|
X-ray synchrotron radiation scattering data from solutions of myelin-associated glycoprotein in 25 mM HEPES, 150 mM NaCl, pH 7.5 were collected on the BM29 camera on the storage ring ESRF (Grenoble, France) using a 2D Photon counting Pilatus 1M pixel detector (I(s) vs s, where s = 4π sin θ/λ; 2θ is the scattering angle). One solute concentration of 3.38 mg/ml was measured from nine successive 2 second frames. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted and the different curves were scaled for protein concentration. The low angle data collected were obtained from a single concentration scattering curve.
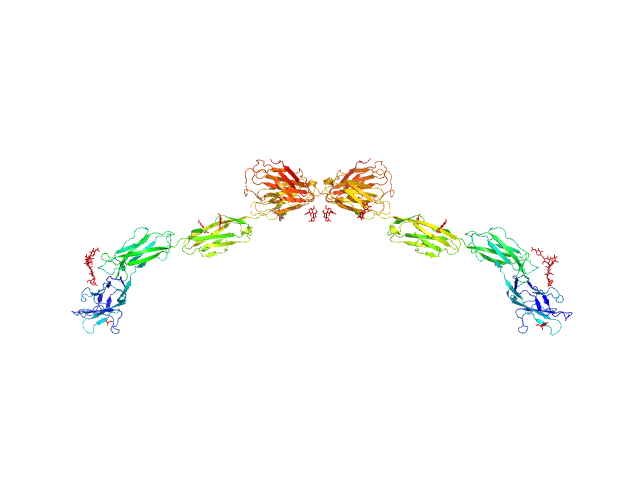
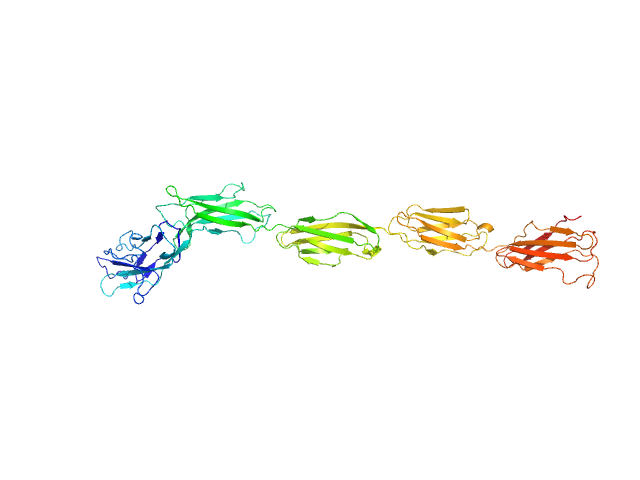
The model fit displayed in this entry represents the volume fraction weighted contributions of the myelin-associated glycoprotein monomer and dimer structures (determined from X-ray crystallography) present in the sample.
|
|
 s, nm-1
s, nm-1