|
Synchrotron SAXS data from solutions of deglycosylated myelin-associated glycoprotein (Ig domains 1-5) in HEPES, pH 7.5 were collected on the BM29 camera on the storage ring ESRF (Grenoble, France) using a Pilatus 1M detector at a sample-detector distance of 2.9 m and at a wavelength of λ = 0.1 nm (I(s) vs s, where s = 4π sin θ/λ and 2θ is the scattering angle). One solute concentration of 2.13 mg/ml was measured at 20°C. 10 successive 2 second frames were collected. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted and the curve was scaled for protein concentration.
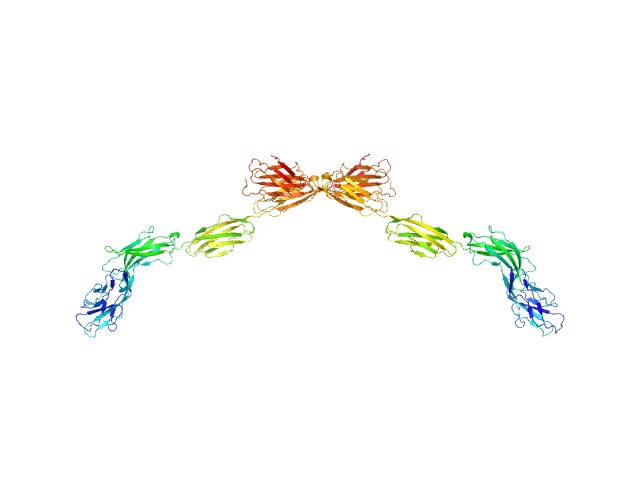
Endo-Hf-deglycosylated full extracellular domain of Myelin-associated glycoprotein (Ig1-5).
|
|
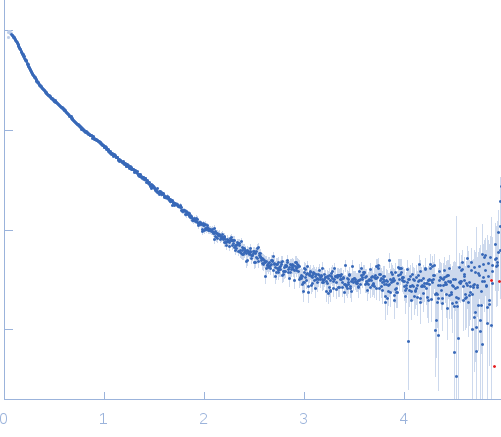 s, nm-1
s, nm-1