|
Synchrotron SAXS data from solutions of Leishmania braziliensis Activator of Hsp90 ATPase-1 in 50 mM sodium phosphate, 50 mM NaCl, 2 mM EDTA, 1 mM β-mercaptoethanol, pH 7, were collected on the SAXS2 Beamline camera on the storage ring Brazilian Synchrotron Light Laboratory (Campinas, Brazil) using a MAR Image Plate detector (I(s) vs s; s = 4π sin θ/λ, where 2θ is the scattering angle and λ=0.1488 nm). Different solute concentrations in the range 1.10-3.20 mg/ml were measured. Three successive 300 second frames were collected. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted and the different curves were scaled for protein concentration. The low angle data collected were merged with the highest concentration high angle data to yield the final composite scattering curve.
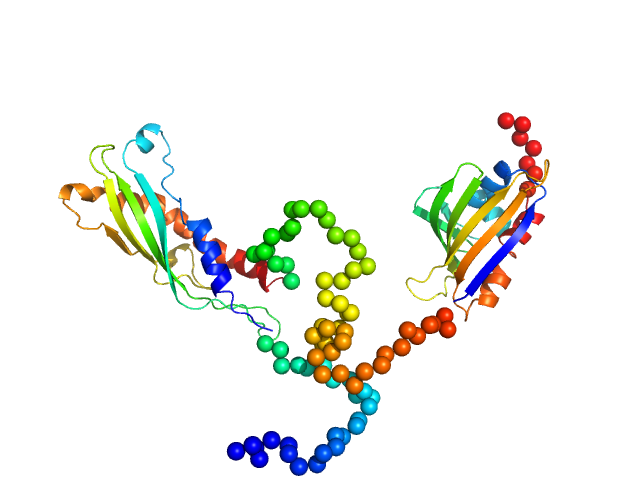
The Ensemble Optimization Method (EOM) model displayed in this entry is a structural representative from a final refined pool of flexible structures. Additional EOM results, including the Rg and Dmax distributions from two EOM modelling runs, are included in the full entry zip archive.
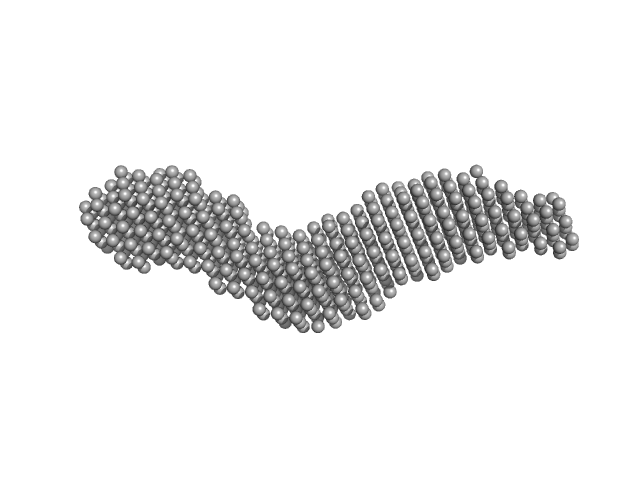
Ab initio model of Leishmania braziliensis Activator of Hsp90 ATPase-1 (LbAha1) and Ensemble Optimization Method analysis on LbAha1 conformational dynamics. Results showed that LbAha1 is remarkably flexible.
|
|
 s, nm-1
s, nm-1
 Rg, nm
Rg, nm