|
Synchrotron SAXS data from solutions of the catalytic domain (AC) of B. Pertussis adenylate cyclase toxin (CyaA) in complex with calmodulin in 20 mM Hepes, 150 mM NaCl, 4 mM CaCl2, pH 7.4 were collected on the SWING beam line at the SOLEIL storage ring (Saint-Aubin, France) using a CCD AVIEX detector at a sample-detector distance of 2.0 m and at a wavelength of λ = 0.1 nm (I(s) vs s, where s = 4πsin θ/λ and 2θ is the scattering angle). 250 successive 1.500 second frames were collected at at 15°C using size-exclusion chromatography SAXS.
The scattered intensities were displayed on an absolute scale (cm-1) using the scattering of water. Frames were examined individually and 20 identical frames were averaged and further processed. The corresponding concentration was 0.82 g/L.
Three independent determinations of the molecular mass were obtained from the value of I(0)/c, where c is the protein concentration, and using the programs SAXSMow2 and ScÅtter3 available at the URLs http://saxs.ifsc.usp.br/ and https://bl1231.als.lbl.gov/scatter/, respectively. The average value is The average value is MWexperimental=56.3 kDa.
AC-CAM complex:
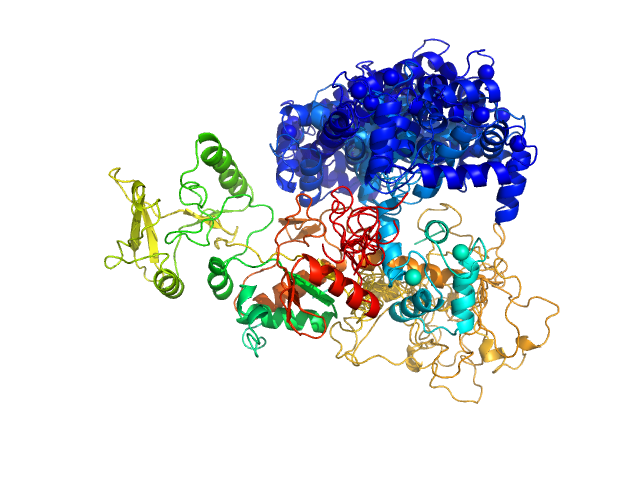
Top panel: Comparison of the experimental data (blue dots) with the calculated scattering pattern (red line) of the BUNCH model shown on the right. chi2=1.96. Each CaM domain were handled as rigid bodies while the program searches an optimal conformation of the inter-domain helix of CaM.
Bottom panel: Typical ensemble of conformations describing the AC:CaM complex, obtained using the program EOM and displayed after superimposition of the AC moiety of each conformation. chi2=1.41. The corresponding scattering curve is shown in red superimposed over experimental data (blue dots).
|
|
 s, nm-1
s, nm-1