|
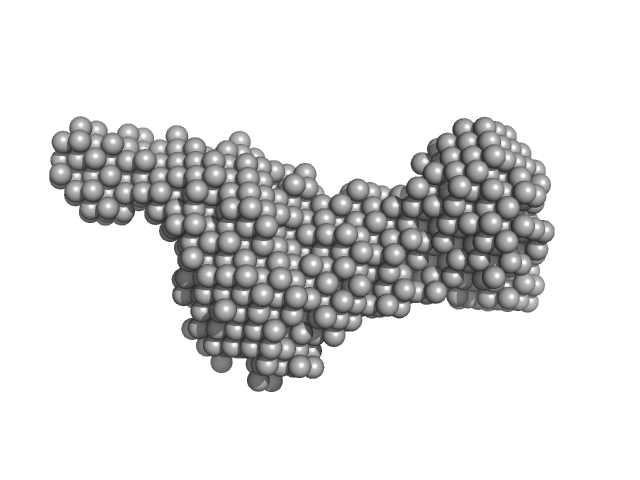
Synchrotron SAXS data from solutions of the tandem CBD from ColG collagenase at pCa 4 in 10 mM HEPES 100 mM NaCL, 2% glycerol, pH 7.5 were collected on the 12.3.1 (SIBYLS) beam line at the Advanced Light Source (ALS, Berkeley, CA, USA) using a Pilatus3 X 2M detector at a sample-detector distance of 1.5 m and at a wavelength of λ = 0.1127 nm (I(s) vs s, where s = 4πsinθ/λ and 2θ is the scattering angle). Solute concentrations ranging between 1 and 5 mg/ml were measured at 10°C. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted and the different curves were scaled for protein concentration. The low angle data collected at lower concentrations were extrapolated to infinite dilution and merged with the higher concentration data to yield the final composite scattering curve.
X-ray Exposure time = UNKNOWN. Number of frames = UNKNOWN
|
|
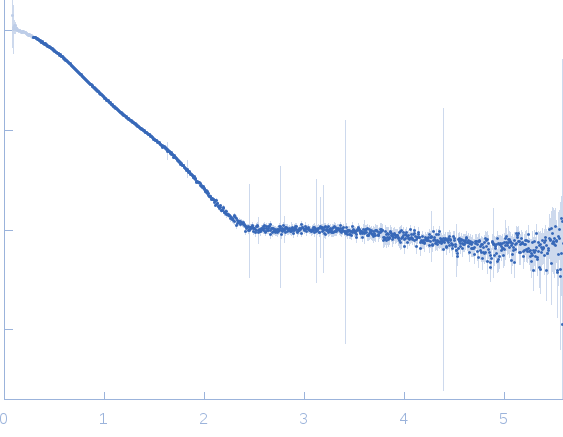 s, nm-1
s, nm-1