|
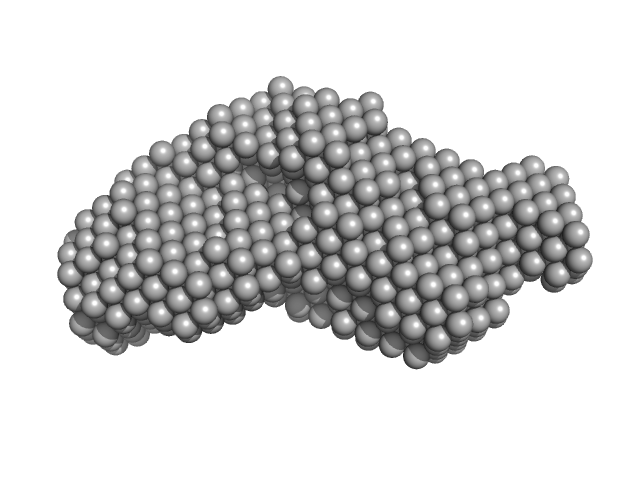
Synchrotron SAXS data from solutions of the C2 domains of human PI3KC2α (Phox homologue (PX)) bound to IP6 in 25 mM Tris 200 mM NaCl 5% v/v glycerol 0.5 mM TCEP 4 mM IP6, pH 8.5, were collected on the SAXS/WAXS camera at the Australian Synchrotron storage ring (Melbourne, Australia) using a Pilatus 1M detector at a wavelength of λ = 0.10332 nm (l(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). In-line size exclusion chromatography SAXS (SEC-SAXS) was used. 15 successive 1 second frames were collected through SEC elution peak of the protein. The data were normalized to the intensity of the transmitted beam, radially averaged. The resulting 1D data frames were then scaled and then averaged. The scattering of an appropriate solvent-blank from the SEC column was subtracted.
SEC-SAXS details: Column type: S200 5/150 GL column; Flow rate: 0.45 ml/min; Sample temperature, 10°C; Sample injection concentration: 10.5 mg/ml. The ab initio models represent the spatially aligned and volume occupancy corrected (averaged) representation of the protein obtained from 20 individual reconstructions (top, DAMFILT model: NSD = 0.66 +/- 0.02; resolution estimate = 3.3 nm) and the best-fit individual DAMMIN reconstruction (bottom) displayed with the corresponding individual model fit to the SAXS data.
|
|
 s, nm-1
s, nm-1