|
Synchrotron SAXS data from solutions of perivitellin (PmPV2) in 20 mM Tris, pH 7 were collected on the SAXS2 beam line at the Brazilian Synchrotron Light Laboratory (Campinas, Brazil) using a MAR 165 CCD detector at a sample-detector distance of 1.5 m and at a wavelength of λ = 0.155 nm (l(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). Solute concentrations ranging between 0.8 and 2 mg/ml were measured at 20°C. Five successive 300 second frames were collected. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted. The low angle data collected at lower concentration were merged with the highest concentration high angle data to yield the final composite scattering curve.
The protein is in the native state and is composed of two dimers of about 95 KDa each. SAXS data curves of different concentrations were merged and analysed using ATSAS 2.8.4-1
|
|
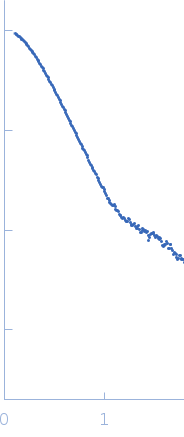 s, nm-1
s, nm-1