|
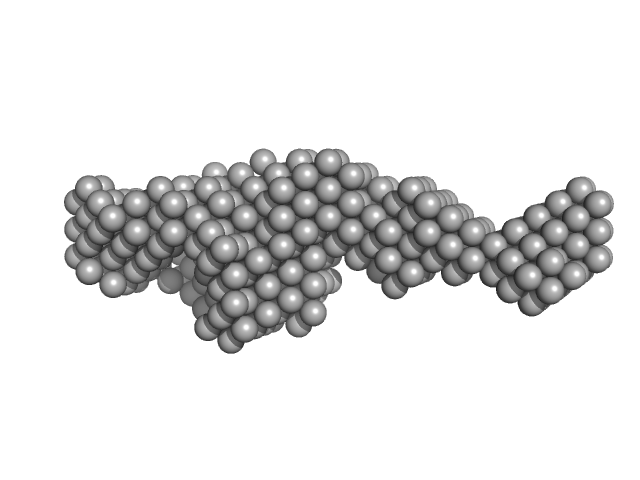
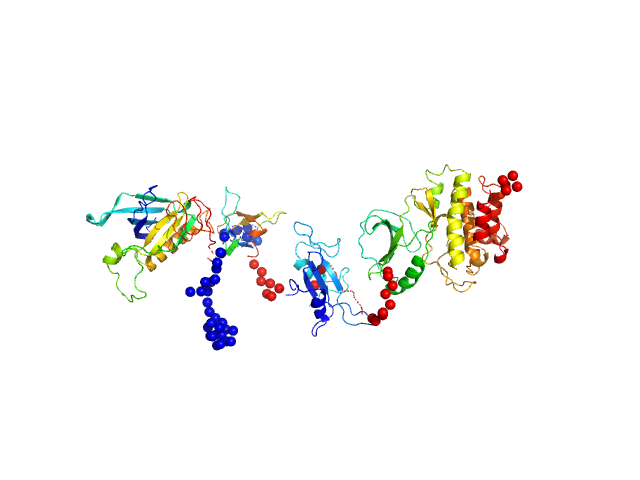
Synchrotron SAXS data for full-length (amino acids 1-659) wild-type of human Bruton’s tyrosine kinase (Btk) in 20 mM Tris-HCl, 150 mM NaCl, 5% Glycerol, 1 mM TCEP, pH 7.5 were collected on the BM29 beam line at the ESRF (Grenoble, France) using a Dectris Pilatus 1M detector at a sample-detector distance of 2.849 m and at a wavelength of λ = 0.099 nm (l(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). Solute concentrations ranging between 0.42 and 5.48 mg/ml were measured at 10°C. 10 successive 0.5 second frames were collected. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted. The low angle data collected at lower concentration were merged with the highest concentration high angle data to yield the final composite scattering curve. The final scattering curve was analysed using PRIMUS software (ATSAS). The model depicts the DAMMIN ab initio reconstruction superimposed with the atomic model SASDC52 (EMBL, Hamburg) subject to SREFLEX to improve its agreement with experimental data. Ensemble optimisation method (EOM) was performed using all four domains structure (2Z0P, 1QLY, 2GE9 and 1K2P, PDB) to assess construct’s flexibility.
|
|
 s, nm-1
s, nm-1
 Rg, nm
Rg, nm