| MWexperimental | 60 | kDa |
| MWexpected | 95 | kDa |
| VPorod | 53 | nm3 |
|
log I(s)
5.99×101
5.99×100
5.99×10-1
5.99×10-2
|
 s, nm-1
s, nm-1
|
|
|
|

|
|
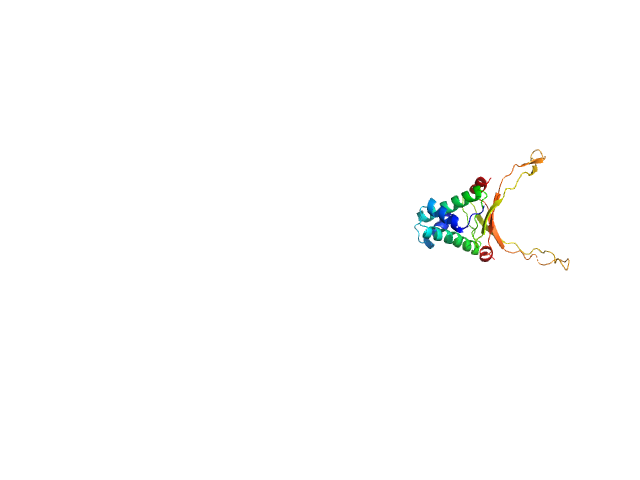
Synchrotron SAXS
data from solutions of
DNA-binding protein HU-alpha, E38K/V42L double mutant
in
50 mM Tris-HCl, 150 mM NaCl, 1 mM DTT, 1 mM PMSF, pH 7.5
were collected
on the
12.3.1 (SIBYLS) beam line
at the Advanced Light Source (ALS) storage ring
(Berkeley, CA, USA)
using a MAR 165 CCD detector
at a sample-detector distance of 1.5 m and
at a wavelength of λ = 0.1 nm
(I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle).
at 10°C.
32 successive
0.100 second frames were collected.
The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
Storage temperature = UNKNOWN. Concentration = UNKNOWN |
|
|||||||||||||||||||||||||||