|
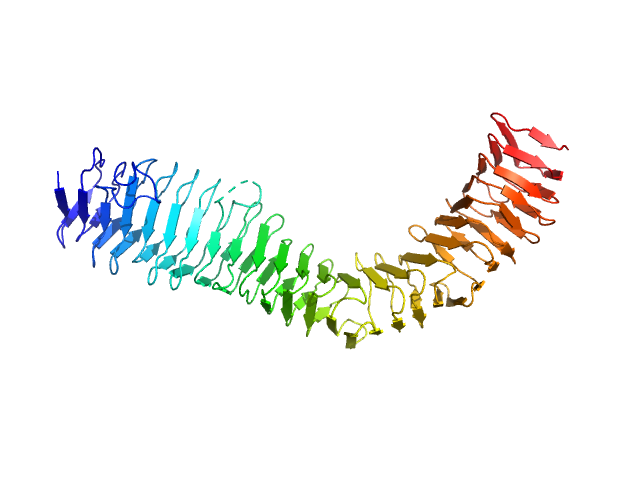
X-ray synchrotron radiation scattering data from Ag43a in 25 mM HEPES 150 mM NaCl, pH 7.0 were collected on the SAXS/WAXS beam line of the Australian Synchrotron (Melbourne, Australia) using a 2D Photon counting Pilatus 1M-W pixel detector (s = 4π sin θ/λ, where 2θ is the scattering angle). Six successive 2 second frames were collected at 3.4 mg/ml. The data were normalized to the intensity of the transmitted beam and radially averaged and the scattering of the solvent-blank was subtracted. The models and corresponding fits include those derived from dummy-atom modelling using DAMMIN, and a fit to the predicted scattering curve of the crystal structure using CRYSOL. Of note, the intensity error estimates are given as 2-standard errors, thus, the Chi values are a factor of two times lower in the log files than is actually the case.
|
|
 s, nm-1
s, nm-1