|
Synchrotron SAXS data from solutions of the HSA-NEP fusion in 10 mM phosphate, pH 6.5 were collected on the EMBL P12 beam line at PETRA III (DESY, Hamburg, Germany) using a Pilatus 2M detector at a sample-detector distance of 3 m and at a wavelength of λ = 0.124 nm (I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). 20 successive 0.050 second frames were collected at 20°C. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted. The low angle data collected at lower concentration (concentration UNKNOWN) were merged with the highest concentration high angle data (concentration UNKNOWN) to yield the final composite scattering curve.
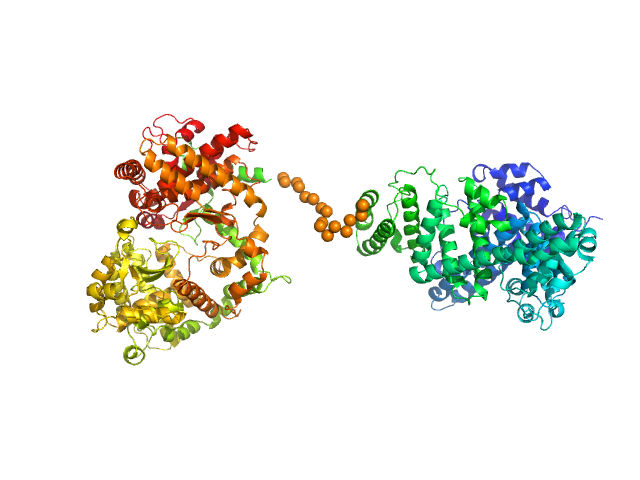
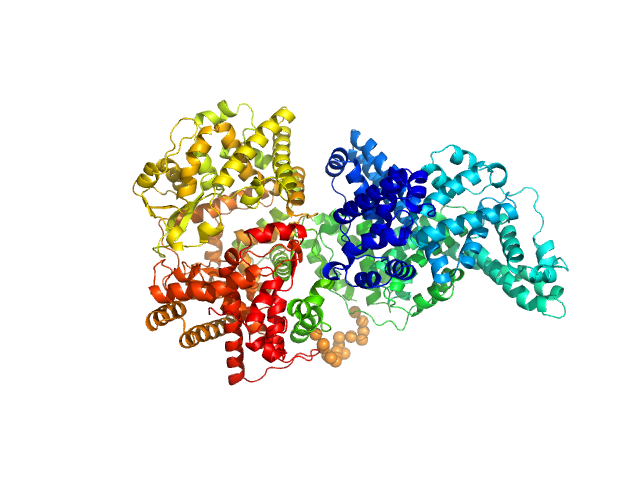
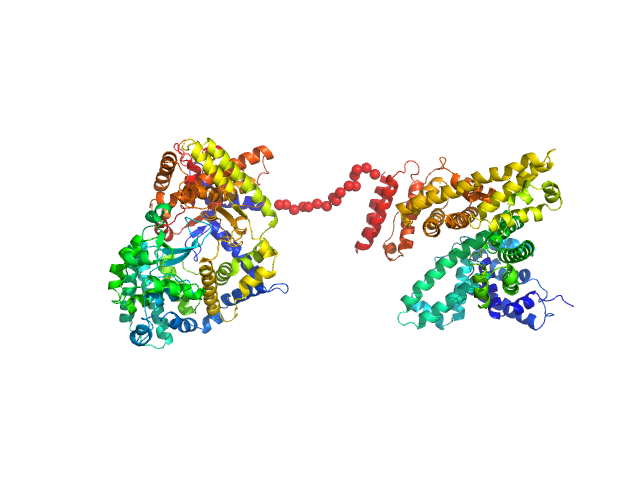
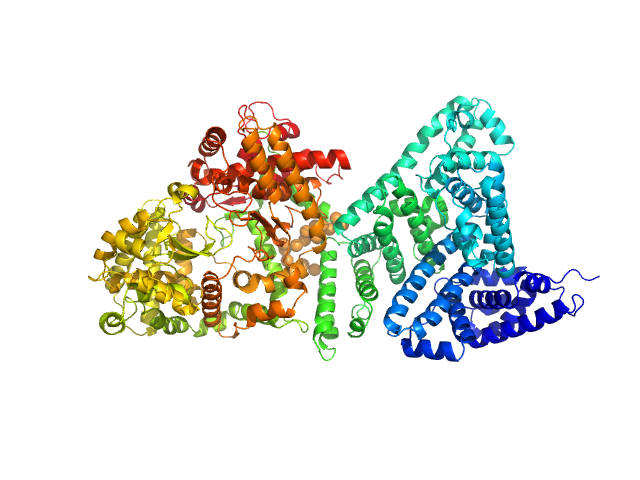
The engineered protein, expressed in Chinese hamster ovary (CHO) cells, is a fusion between HSA and human Neprilysin connected by a GGGGS amino acid linker. CAUTION! The Guinier region of the data has been entirely removed for ensemble optimization method (EOM) modelling calculations. The cation on the 'phosphate' is unspecified.
|
|
 s, nm-1
s, nm-1