|
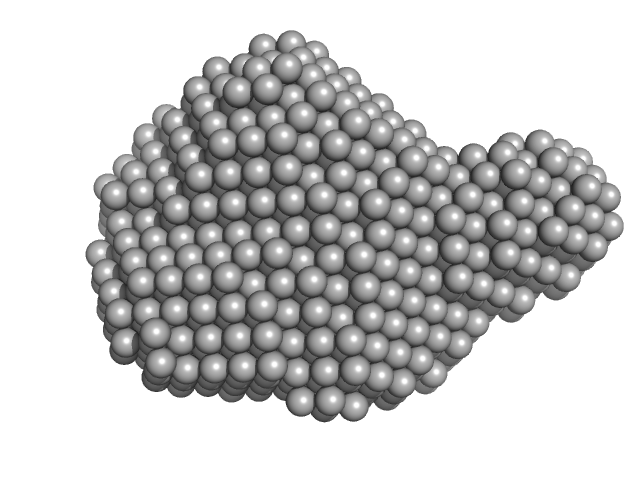
Synchrotron SAXS data from solutions of Protein Disulfide Isomerase (PDI) in complex with Ribonuclease-A (RNase) in 50 mM Tris-HCL, 150 mM NaCl, pH 7.5 were collected on the BioCAT 18ID beam line at the Advanced Photon Source (APS), Argonne National Laboratory storage ring (Lemont, IL, USA) using a Pilatus3 X 1M detector at a sample-detector distance of 3.5 m and at a wavelength of λ = 0.1033 nm (I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). Solute concentrations ranging between 2.5 and 10 mg/ml were measured at 10°C. Eight successive 1 second frames were collected. Using the automated data processing pipeline at the beam line, the data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted. The low angle data collected at lower concentration were merged with the highest concentration high angle data to yield the final composite scattering curve. Subsequent data processing and three-dimensional modeling were performed using the ATSAS [2.8.3] package.
PDI was purified from bovine liver. Ribonuclease A (Type III-A) was purchased from SIGMA-ALDRICH in the form of lyophilized powder, dissolved with suspension medium and used directly without any further purification. RNase was mixed with PDI just before to SAXS data collection.
CAUTION! The quoted experimental MW from standardized I(0) and concentration = 56 kDa; From Bayesian inference = 44 kDa; From Porod volume = 53 kDa. The expected MW of a fully-formed 1:1 complex , calculated from the amino acid sequence, is 71 kDa.
|
|
 s, nm-1
s, nm-1