|
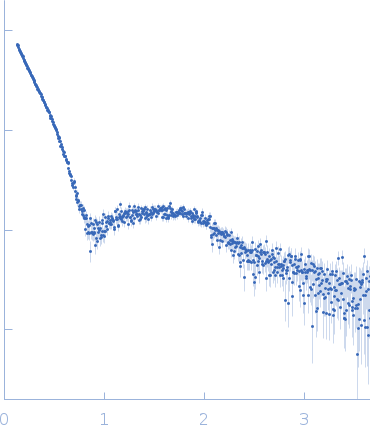
Synchrotron SAXS
data from solutions of
Sensory rhodopsin II - transducer complex (NpSRII/NpHtrII) in detergent at 150 mM NaCl studied with SAXS
in
150 mM NaCl, 25 mM Na/Na-Pi, 1.0 mM EDTA, 0.05% DDM, pH 8
were collected
on the
BM29 beam line
at the ESRF storage ring
(Grenoble, France)
using a Pilatus 1M detector
at a sample-detector distance of 2.9 m and
at a wavelength of λ = 0.9918 nm
(I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle).
Solute concentrations ranging between 0.5 and 0.6 mg/ml were measured
at 20°C.
16 successive
1 second frames were collected.
The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
The low angle data collected at lower concentration were merged with the highest concentration high angle data to yield the final composite scattering curve.
The protein of study is a non-fused complex of Sensory rhodopsin II (NpSRII, UniProt ID P42196) with its cognate transducer (NpHtrII, UniProt ID P42259) from Nartonomonas pharaonis. NpSRII/NpHtrII requires dimerization for signal transduction and forms a trimer of dimers in the N. pharaonis membrane. The dependence of the oligomeric state of this complex on conditions is the subject of research. The NpSRII/NpHtrII was co-expressed in E. coli and solubilized in n-Dodecyl β-D-maltoside; therefore, resulting molecular weight may differ from the expected due to the presence of a detergent belt surrounding the tramembrane domain of the complex.
|
|
 s, nm-1
s, nm-1




