|
SAXS profiles for structural modeling of the HtrA2 hexamer were obtained at the SIBYLS beamline of the Advanced Light Source at Lawrence Berkeley National Laboratory. Measurements of S306A HtrA2 were performed in a buffer containing 20 mM HEPES-NaOH (pH 7.4), 120 mM NaCl, 1 mM EDTA, and 2% glycerol, with four different protein concentrations (1, 2, 5, and 10 mg/mL). Datasets were collected at 23-26 oC with 0.3 sec exposures for 10 sec, and a buffer blank was subtracted from each exposure. Each buffer-subtracted exposure was checked for radiation damage and the exposures without significant radiation damage were merged to produce a final curve using the SAXS FrameSlice online server (https://sibyls.als.lbl.gov/ran/). The radii of gyration (Rg) were obtained using the AUTORG module implemented in PRIMUS. Rigid-body docking was performed using the SASREFMX program. A starting HtrA2 structure (trimer) was used, generated by filling in the disordered loops using the SWISS-MODEL server, with the available crystal structure of the HtrA2 trimer as a template (PDB ID: 1LCY). In the docking calculation a distance restraint of 7Å between the Y451 Cα atom of one trimer and any Cα atom of a second trimer was used; otherwise standard settings were employed.
X-ray wavelength: UNKNOWN. Sample to detector distance: UNKNOWN.
|
|
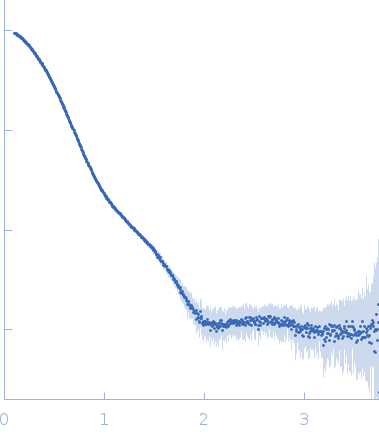 s, nm-1
s, nm-1
