|
Synchrotron SAXS data from solutions of LRSAM1 (amino acids 80-171) in 50 mM Tris-HCl pH 8.0, 150 mM NaCl, 1 mM TCEP, pH 8 were collected on the 4C beam line at the Pohang Accelerator Laboratory (Pohang, South Korea) using a Rayonix SX165 detector at a sample-detector distance of 3 m and at a wavelength of λ = 0.0734 nm (I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). Solute concentrations ranging between 1.7 and 4.6 mg/ml were measured at 20°C. Six successive 10 second frames were collected. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted. The low angle data collected at lower concentrations were extrapolated to infinite dilution and merged with the higher concentration data to yield the final composite scattering curve.
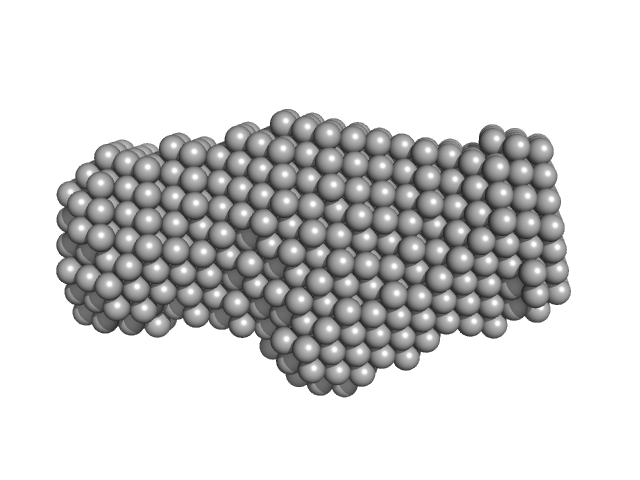
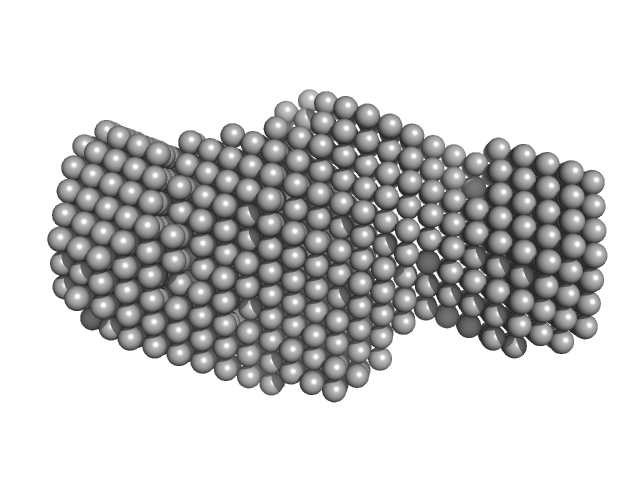
The models displayed show both the average-weighted bead-occupancy and volume-corrected representation of the protein calculated from the spatial alignment of a cohort of individual models (damfilt; top) and an individual model reconstruction (bottom) with the associated fit to the data.
|
|
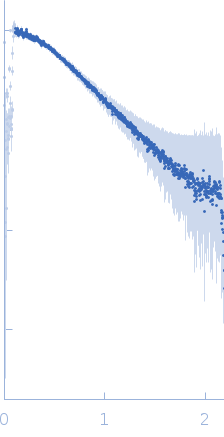 s, nm-1
s, nm-1