|
Synchrotron SAXS
data from solutions of
Amyloid Beta 1-42 (cluster 2, Set 4, initial state)
in
1 mM Hepes, 0.1 % NH4OH (pH∼10.7), pH 10.7
were collected
on the
EMBL P12 beam line
at the PETRA III storage ring
(DESY; Hamburg, Germany)
using a Pilatus 6M detector
at a sample-detector distance of 3.1 m and
at a wavelength of λ = 0.124 nm
(I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle).
One solute concentration of 2.00 mg/ml was measured
at 20°C.
40 successive
0.095 second frames were collected.
The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
SAXS curve captured during the fibril formation process. This curve was used to obtain and characterise the structural kinetics of cluster 2 studying the process of fibrils formation with TR-SAXS. The quoted 'expected MW' is that calculated from the amino acid sequence of the monomeric form of the protein.
|
|
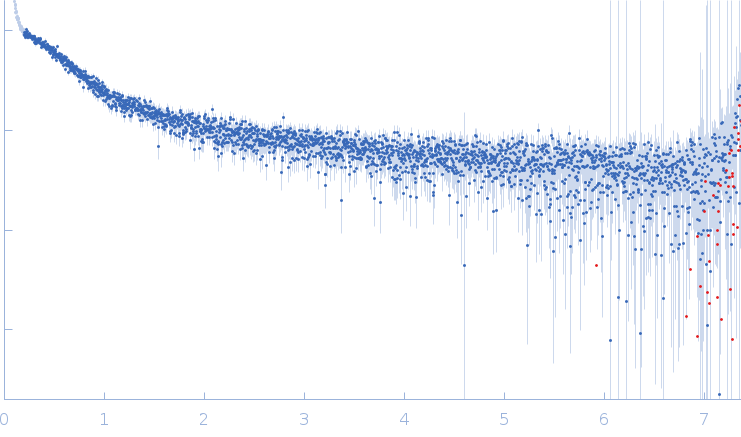 s, nm-1
s, nm-1