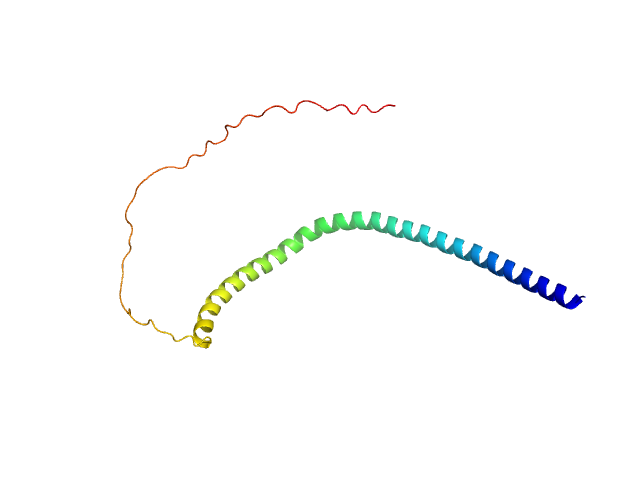
|
SAXS data from solutions of alpha synuclein in 10 mM HEPES, 50 mM NaCl, pH 7.4 were were collected using an Anton Paar SAXSpace at the CSIR Institute of Microbial Technology (IMTech; Chandigarh, India) equipped with a Mythen 1K detector at a sample-detector distance of 0.3 m and at a wavelength of λ = 0.15414 nm (I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). One solute concentration of 8.00 mg/ml was measured at 10°C. Three successive 1800 second frames were collected. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
CAUTION: Experimental errors are incorrectly specified (negative values). CAUTION: Experimental molecular weight cannot be verified from the data. The human wild-type α-syn protein was expressed in E. coli Rosetta 2 (DE3) using pT7-7 based
expression vector. The protein expression was induced using 0.3mM IPTG for 8h at 30 °C. Cells were
harvested by centrifugation and further resuspended into buffer containing in10 mM HEPES, 50 mM
NaCl, (pH 7.4). The harvested cell pellet was stored at -80 °C until further use. Briefly, cell lysis was
carried out by sonication, and cellular lysate was boiled at 95 °C for 30 minutes. Streptomycin sulfate
and Glacial acetic acid precipitated nucleic acid material was removed by centrifugation at 12000
r.p.m. for 30 minutes at 4 °C. The α-syn was precipitated by addition of 50% ammonium sulphate
followed by incubation on ice for 1 h with regular shaking after every 10 minutes. The pellet was
separated, and further washed with equal volume of 100 mM ammonium acetate followed by equal
volume of ethanol. The washed protein pellet was dried to evaporate any residual ethanol and
further dissolved in10 mM HEPES, 50 mM NaCl, pH 7.4 and extensively dialyzed to remove
ammonium sulphate. Protein purity was confirmed on 15% SDS-PAGE.
|
|
 s, nm-1
s, nm-1