|
The Ig domain from myelin protein zero (IgMPZ) was exchanged into SAXS buffer (50 mM NaCl, 1 mM EDTA, 20 mM TrisCl pH 7.6) and concentrated to 105 µM. A second 52 µM was generated by dilution with SAXS buffer. The final 50 µl samples were centrifuged for five minutes at 13,000 rpm. SAXS was performed at BioCAT (beamline 18ID at the Advanced Photon Source, Chicago) with in-line sample loading that was run at 0.3 ml/min on an AKTA Pure FPLC (GE) without column separation. The flow cell consists of a 1.0 mm ID quartz capillary with ~20 µm walls. A coflowing buffer sheath was used to separate sample from the capillary walls and to help prevent radiation damage. Scattering intensity was recorded using an Eiger2 XE 9M (Dectris) detector which was placed 3.65 m from the sample giving us access to an s-range of 0.03 nm-1 to 4.2 nm-1. 0.25 s exposures were acquired every 0.5 s during elution.
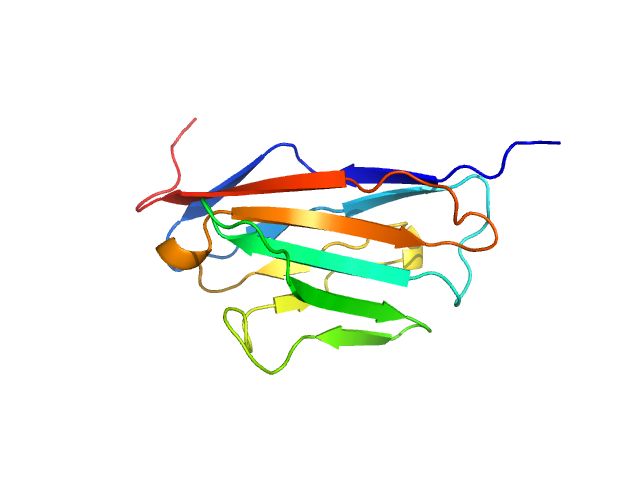
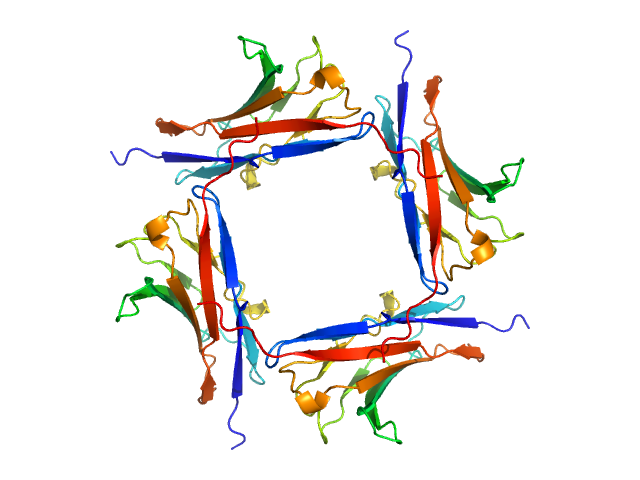
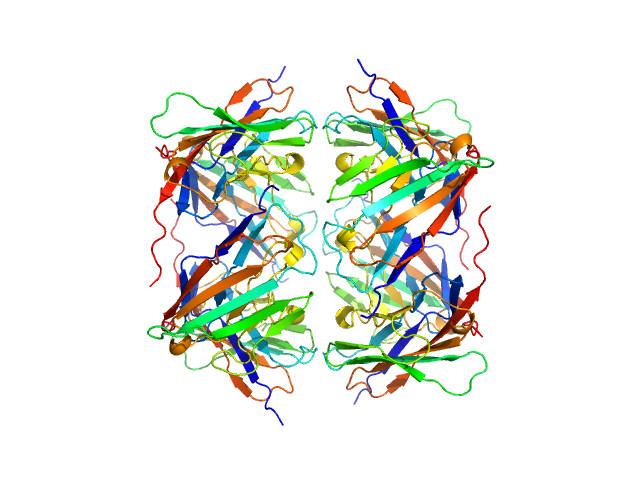
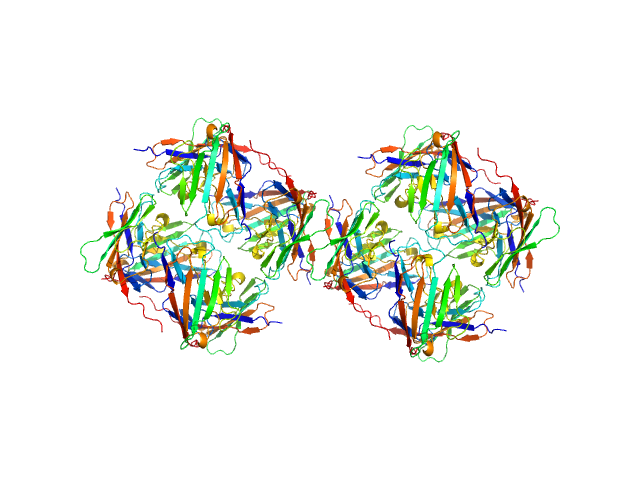
Data was reduced using BioXTAS RAW 2.1.1. I(s) vs s scattering curves were created from exposures selected from the sample peak. Matching buffer blanks for background subtraction were obtained from averaged pre-sample exposures. The fraction of oligomers was determined from experimental SAXS data using the OLIGOMER program from the ATSAS suite. Homo-oligomeric arrangements of IgMPZ were identified from the expanded unit cell of the IgMPZ crystal structure (PDB ID: 1NEU) generated in UCSF Chimera. To obtain theoretical form factors, structural predictions for the final proteins from our expressed human IgMPZ constructs were generated by a ColabFold search of MMseqs2 with AlphaFold2. Oligomeric arrangements were generated by superimposition of ColabFold IgMPZ structures onto crystal structure-derived IgMPZ oligomers. Five oligomers (volume fractions) were used in the OLIGOMER fitting: 2-mer (0.178), 1-mer (0.512), 4-mer (0.218), 8-mer (0.060), 16-mer (0.032).
Additional concentration series data and mixture analysis are made available in the full entry zip archive. At 52 μM, volume fractions used in the OLIGOMER fitting were 2-mer (0.053), 1-mer (0.672), 4-mer (0.173), 8-mer (0.079), 16-mer (0.023).
|
|
 s, nm-1
s, nm-1