|
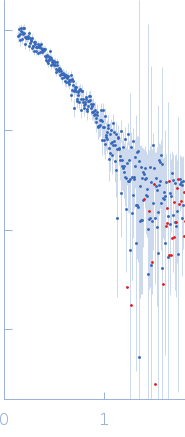
SAXS data from solutions of a G- / I-actin mixture from Oryctolagus cuniculus (a mixture of globular actin monomers and inactivated actin monomers/dimers) in 5 mM Tris, 0.1 mM CaCl2, 1 mM NaN3, 0.2 mM ATP, pH 8.1 were collected using a Rigaku MicroMax 007-HF instrument at the Moscow Institute of Physics and Technology (MIPT; Dolgoprudny, Russian Federation) equipped with a multiwire gas-filled ASM DTR Triton 200 detector at a sample-detector distance of 2 m and at a wavelength of λ = 0.15406 nm (I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). One solute concentration of 1.25 mg/ml was measured at 20°C. One 3600 second frame was collected. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
One week incubation of G-actin leads to formation of mixture of G-actin monomers and I-actin monomers and dimers. The exact structures of I-actin monomers and dimers are unknown. However, using the values of Rg and Vp, volume fraction of dimers and distance Dm-m between the centres of two monomers in these dimers we found to be equal to 4.6 ± 2.1, and 5.1 ± 1.4 nm, respectively.
|
|
 s, nm-1
s, nm-1
![Static model image Actin, alpha skeletal muscle OTHER [STATIC IMAGE] model](/media//pdb_file/SASDT25_fit1_model1.png)