|
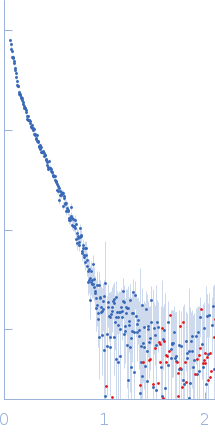
SAXS data from solutions of F-actin from Oryctolagus cuniculus (fibrillar actin) in 5 mM Tris/Tris-HCl, 0.1 mM CaCl2, 1 mM NaN3, 1.0 mM ATP, 50 mM KCl, 2 mM MgCl2, pH 8.1 were collected using a Rigaku MicroMax 007-HF instrument at the Moscow Institute of Physics and Technology (MIPT; Dolgoprudny, Russian Federation) equipped with a multiwire gas-filled ASM DTR Triton 200 detector at a sample-detector distance of 2 m and at a wavelength of λ = 0.15406 nm (I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). Solute concentrations ranging between 0.5 and 6.3 mg/ml were measured at 20°C. Five successive 1800 second frames were collected. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
The best approximation for F-actin SAXS data, based on simple shaped models in SasView program, is achieved by the semi-flexible elliptical cylinder. Major and minor ellipse axes are 8.5 nm and 5.2 nm, which is in good agreement with the dimensions of the known F-actin structure (PDB ID: 3J8I). SAXS data for F-actin are also well-fitted by DAMMIF program, using assumption of “prolate” particle. The transverse dimensions of the ab initio model coincide with those in 3J8I structure.
|
|
 s, nm-1
s, nm-1

![Static model image Actin, alpha skeletal muscle OTHER [STATIC IMAGE] model](/media//pdb_file/SASDTY4_fit2_model1.png)