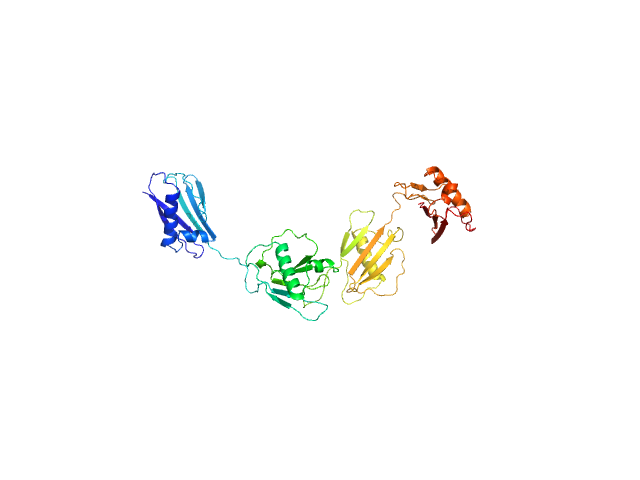
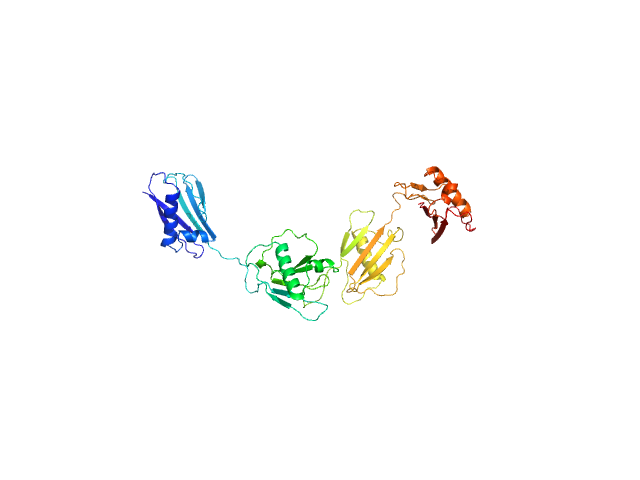
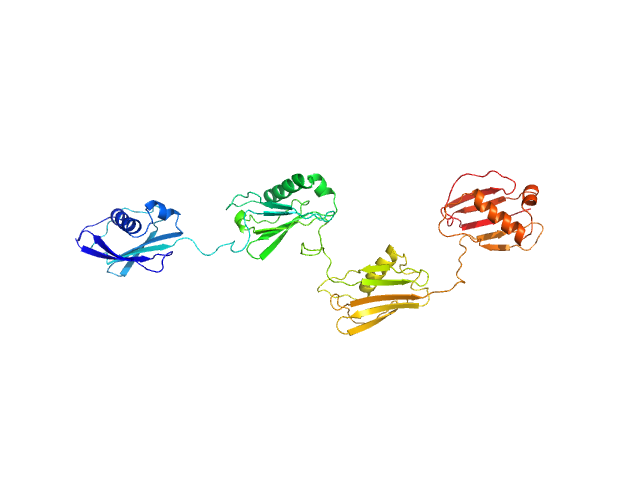
| MWI(0) | 55 | kDa |
| MWexpected | 50 | kDa |
| VPorod | 64 | nm3 |
|
log I(s)
5.31×101
5.31×100
5.31×10-1
5.31×10-2
|
 s, nm-1
s, nm-1
|
|
|
|
|
|
|
|
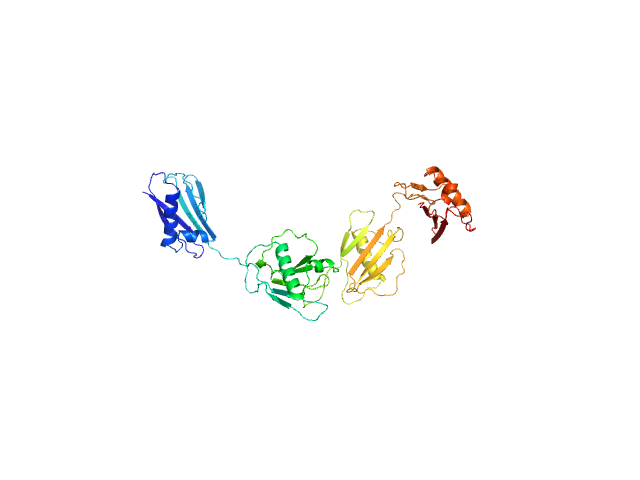
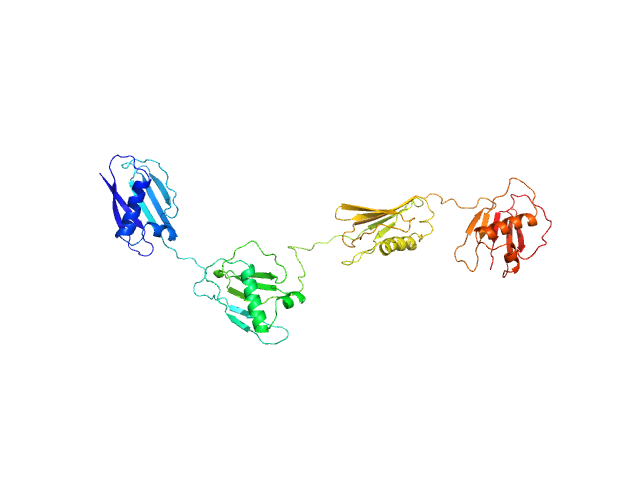
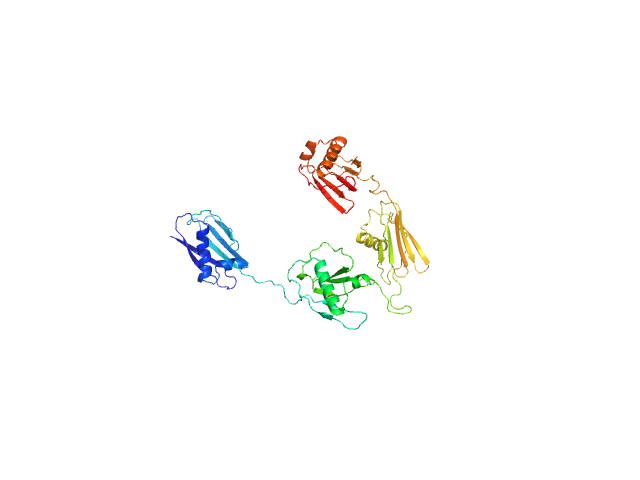
|
|
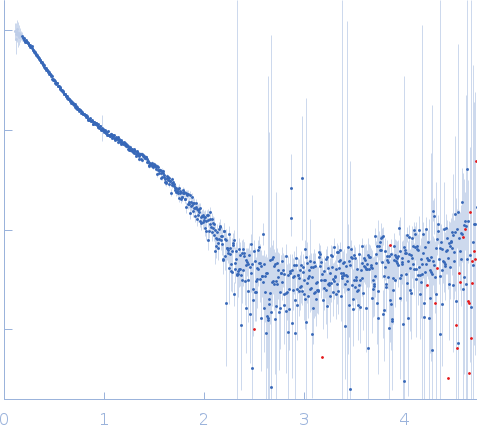
Synchrotron SAXS
data from solutions of
Staphylococcal extracellular adherence protein (Eap) in unbound form
in
20 mM HEPES, 140 mM NaCl, pH 7.4
were collected
on the
12.3.1 (SIBYLS) beam line
at the Advanced Light Source (ALS) storage ring
(Berkeley, CA, USA)
using a Pilatus3 X 2M detector
at a sample-detector distance of 2.1 m and
at a wavelength of λ = 0.1127 nm
(I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle).
In-line size-exclusion chromatography (SEC) SAS was employed. The SEC parameters were as follows: A 60.00 μl sample
at 5 mg/ml was injected at a 0.65 ml/min flow rate
onto a Shodex LW-803 column
at 4°C.
660 successive
2 second frames were collected.
The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
|
|
|||||||||||||||||||||||||||