|
Synchrotron SAXS data from solutions of the single alpha-helix (SAH) region of Drebrin in 17 mM NaH2PO4, 3 mM Na2HPO4, 50 mM NaCl, pH 6 were collected on the EMBL P12 beam line at PETRA III (DESY; Hamburg, Germany) using a Pilatus 6M detector at a sample-detector distance of 3 m and at a wavelength of λ = 0.123982 nm (I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). One solute concentration of 3.20 mg/ml was measured at 20°C. 20 successive 0.100 second frames were collected. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
The single alpha helix ensemble displayed in this entry consists of 60 individual models informed by NMR chemical-shift results and are made available for download in the full-entry zip archive. For display purposes, the models have been aligned relative to amino acids 190-223 (UniProt amino acid numbering. Alternatively, amino acids 26-59, NMR model numbering). The fit to the SAXS data of the ensemble was determined using Pepsi-SAXS. Batch SAXS data spanning a three point concentration series (where the data have been normalised to protein concentration) at 0.82, 1.64 and 3.2 mg/ml are also provided in the full-entry zip archive in addition to SEC-MALLS/RI measurements. NOTE: The experimental molecular weight quoted in this entry is derived from this SEC-MALLS/RI analysis (11 kDa).
|
|
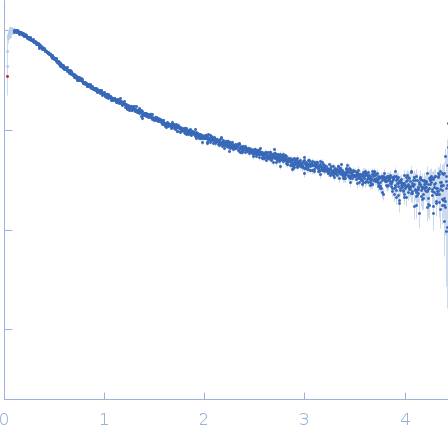 s, nm-1
s, nm-1
![Static model image Drebrin OTHER [STATIC IMAGE] model](/media//pdb_file/SASDVV6_fit1_model1.png)