Structure and mechanism of a phage-encoded SAM lyase revises catalytic function of enzyme family.
Guo X,
Söderholm A,
Kanchugal P S,
Isaksen GV,
Warsi O,
Eckhard U,
Trigüis S,
Gogoll A,
Jerlström-Hultqvist J,
Åqvist J,
Andersson DI,
Selmer M
Elife
10
(2021 Feb 10)
|
|
|
|
|
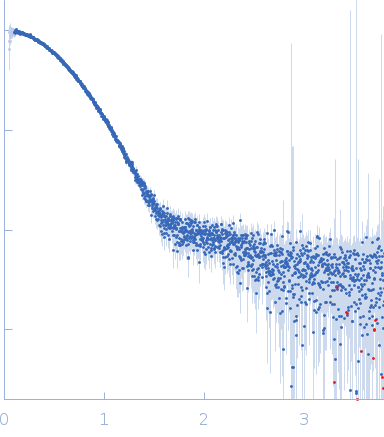
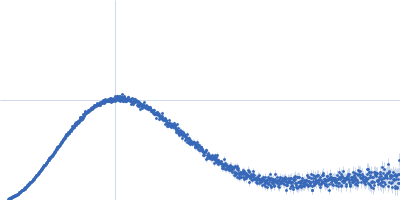
| Sample: |
Phage-encoded SAM lyase Svi3-3 (including N-terminal His6-tag and Tev cleavage site) trimer, 56 kDa Unknown environmental phage protein
|
| Buffer: |
25 mM Tris-HCl, 150 mM NaCl, pH: 8 |
| Experiment: |
SAXS
data collected at B21, Diamond Light Source on 2016 Dec 9
|
|
| RgGuinier |
2.5 |
nm |
| Dmax |
9.3 |
nm |
| VolumePorod |
85 |
nm3 |
|
|
|
|
|
|
|
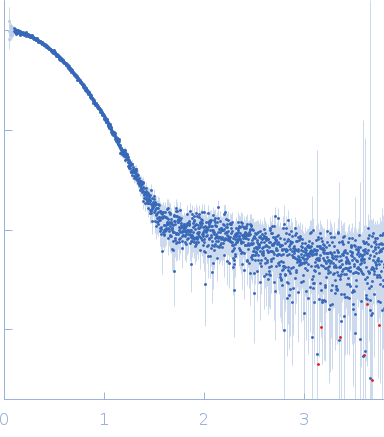
| Sample: |
Phage-encoded SAM lyase Svi3-3 (including N-terminal His6-tag and Tev cleavage site) trimer, 56 kDa Unknown environmental phage protein
|
| Buffer: |
25 mM Tris-HCl, 150 mM NaCl, 5 mM SAM, pH: 8 |
| Experiment: |
SAXS
data collected at B21, Diamond Light Source on 2016 Dec 9
|
|
| RgGuinier |
2.5 |
nm |
| Dmax |
9.1 |
nm |
| VolumePorod |
89 |
nm3 |
|
|