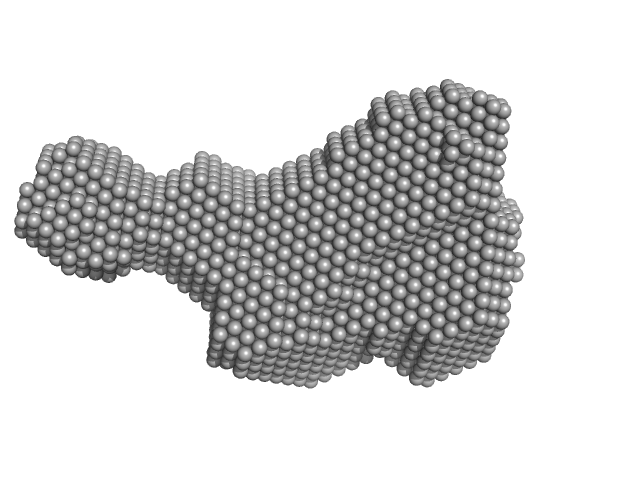
Modulation of the substrate specificity of the kinase PDK1 by distinct conformations of the full-length protein
Sacerdoti M,
Gross L,
Riley A,
Zehnder K,
Ghode A,
Klinke S,
Anand G,
Paris K,
Winkel A,
Herbrand A,
Godage H,
Cozier G,
Süß E,
Schulze J,
Pastor-Flores D,
Bollini M,
Cappellari M,
Svergun D,
Gräwert M,
Aramendia P,
Leroux A,
Potter B,
Camacho C,
Biondi R
Science Signaling
16(789)
(2023 Jun 13)
|
|
|
|
|
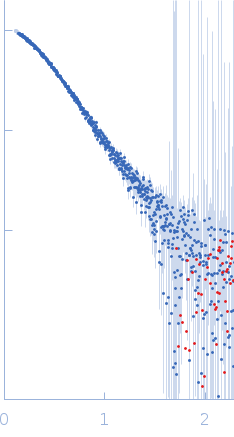
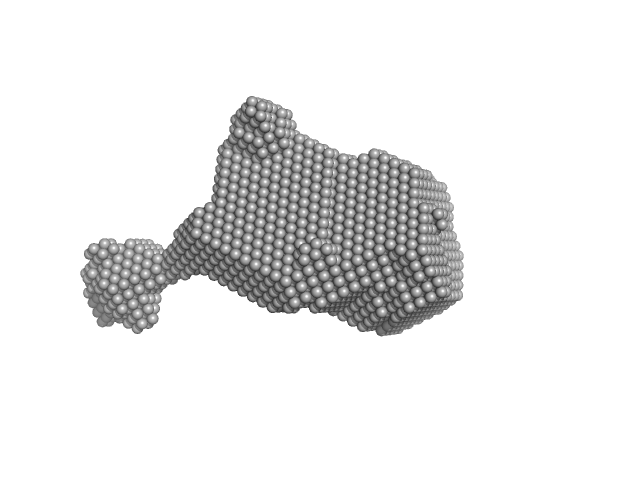
| Sample: |
3-phosphoinositide-dependent protein kinase 1 monomer, 65 kDa Homo sapiens protein
|
| Buffer: |
20 mM Tris-HCl pH 7.4, 250 mM NaCl, 1 mM DTT, pH: 7.4 |
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2021 Mar 26
|
|
| RgGuinier |
3.5 |
nm |
| Dmax |
11.2 |
nm |
| VolumePorod |
107 |
nm3 |
|
|
|
|
|
|
|
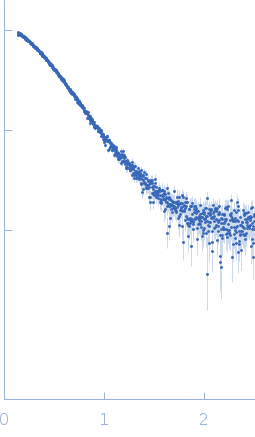
| Sample: |
3-phosphoinositide-dependent protein kinase 1 monomer, 65 kDa Homo sapiens protein
2-O-benzoyl-Ins(1,3,4,5,6)P5 monomer, 1 kDa synthetic construct
|
| Buffer: |
20 mM Tris-HCl pH 7.4, 250 mM NaCl, 1 mM DTT, 1 μM HYG8, pH: 7.4 |
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2021 Mar 26
|
|
| RgGuinier |
3.5 |
nm |
| Dmax |
11.5 |
nm |
| VolumePorod |
98 |
nm3 |
|
|
|
|
|
|
|
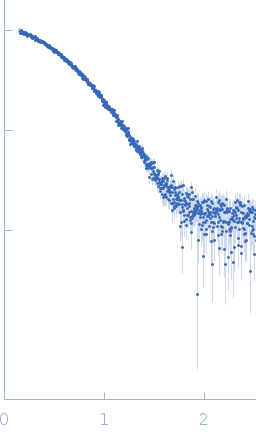
| Sample: |
3-phosphoinositide-dependent protein kinase 1 (Y188G Q292A) monomer, 35 kDa Homo sapiens protein
|
| Buffer: |
20 mM Tris-HCl pH 7.4, 250 mM NaCl, 1 mM DTT, pH: 7.4 |
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2021 Mar 26
|
|
| RgGuinier |
2.4 |
nm |
| Dmax |
7.0 |
nm |
| VolumePorod |
57 |
nm3 |
|
|