Structural insights into the C-terminus of the histone-lysine N-methyltransferase NSD3 by small-angle X-ray scattering.
Belviso BD,
Shen Y,
Carrozzini B,
Morishita M,
di Luccio E,
Caliandro R
Front Mol Biosci
11:1191246
(2024)
|
|
|
|
|
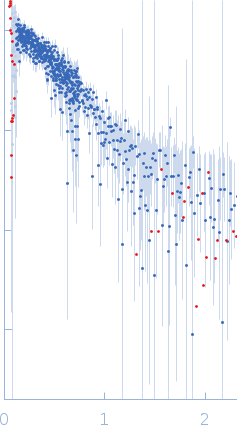
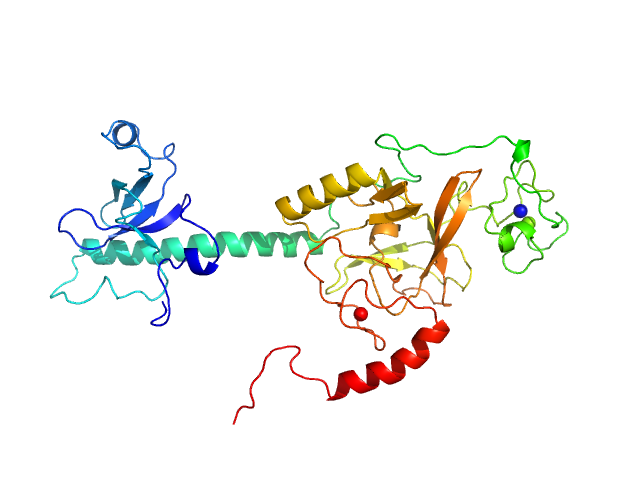
| Sample: |
Histone-lysine N-methyltransferase NSD3 monomer, 43 kDa Homo sapiens protein
|
| Buffer: |
0.5 M NaCl, 20 mM Tris-HCl, 5 mM DTT, pH: 8.5 |
| Experiment: |
SAXS
data collected at B21, Diamond Light Source on 2019 Nov 25
|
|
| RgGuinier |
3.1 |
nm |
| Dmax |
11.2 |
nm |
| VolumePorod |
64 |
nm3 |
|
|
|
|
|
|
|
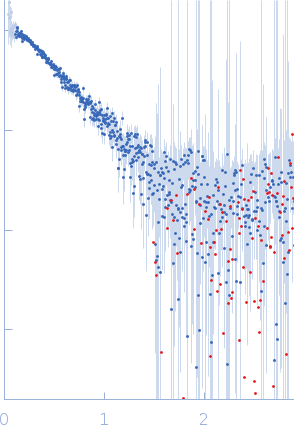
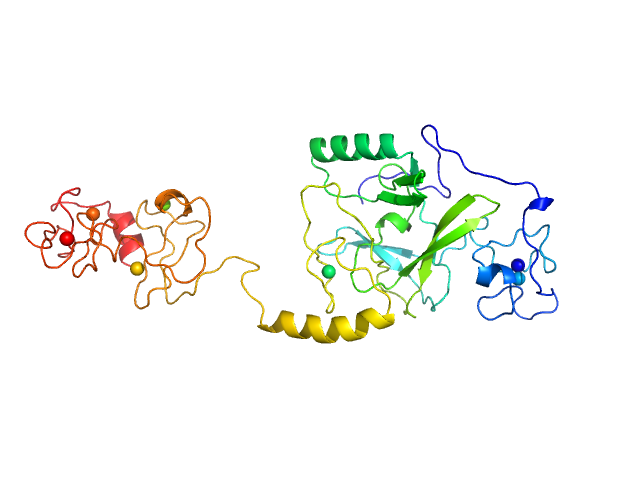
| Sample: |
Histone-lysine N-methyltransferase NSD3 monomer, 40 kDa Homo sapiens protein
|
| Buffer: |
0.5 M NaCl, 20 mM Tris-HCl, 5 mM DTT, pH: 8.5 |
| Experiment: |
SAXS
data collected at B21, Diamond Light Source on 2019 Nov 25
|
|
| RgGuinier |
3.4 |
nm |
| Dmax |
13.2 |
nm |
| VolumePorod |
75 |
nm3 |
|
|