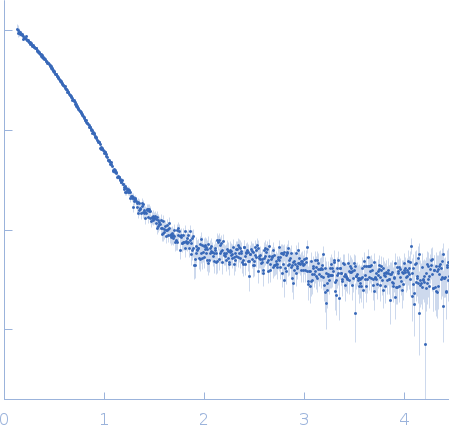
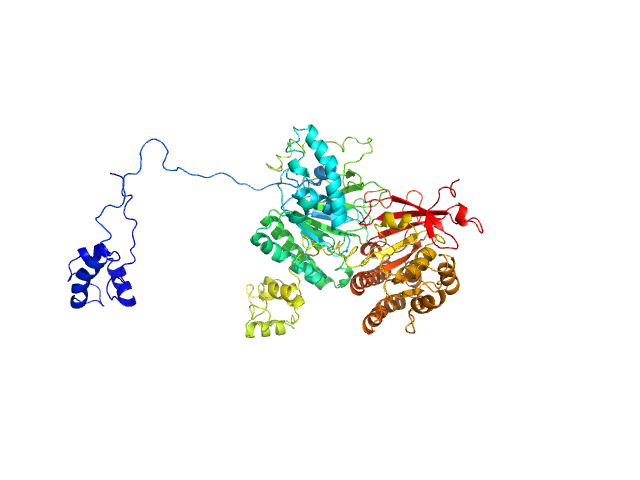
RAD51C-XRCC3 structure and cancer patient mutations define DNA replication roles
Longo M, Roy S, Chen Y, Tomaszowski K, Arvai A, Pepper J, Boisvert R, Kunnimalaiyaan S, Keshvani C, Schild D, Bacolla A, Williams G, Tainer J, Schlacher KNature Communications 14(1) (2023 Jul 24)
|
doi: 10.1038/s41467-023-40096-1 |
Submitted to SASBDB: 2023 Jun 17 Published in SASBDB: |
|
|||||||||||||||||||||||