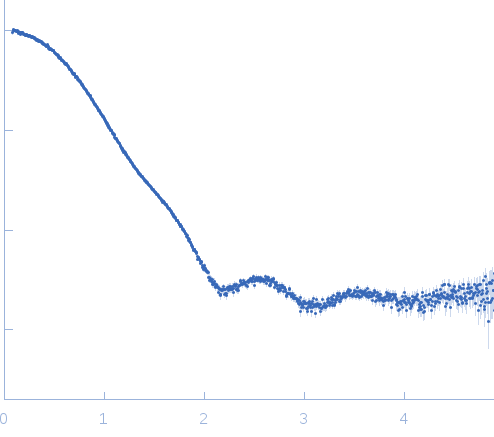
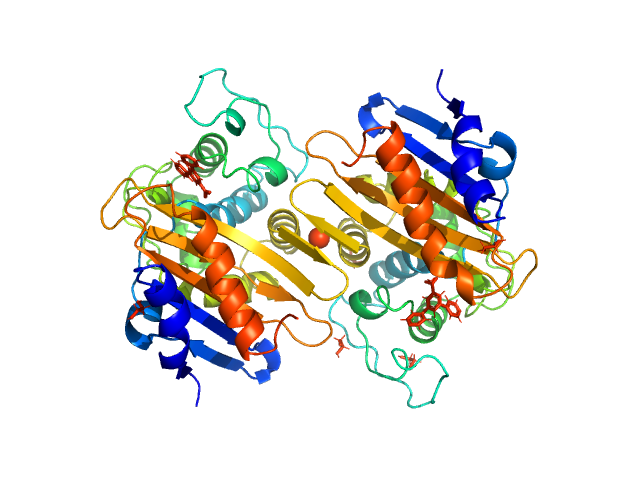
The biological assembly of OXA-48 reveals a dimer interface with high charge complementarity and very high affinity.
Lund BA, Thomassen AM, Nesheim BHB, Carlsen TJO, Isaksson J, Christopeit T, Leiros HSFEBS J (2018 Aug 28)
|
PMID: 30153368
doi: 10.1111/febs.14643 |
Submitted to SASBDB: 2018 Aug 30 Published in SASBDB: |
|
|||||||||||||||||||||||