Thermodynamics, cooperativity and stability of the tetracycline repressor (TetR) upon tetracycline binding.
Palm GJ,
Buchholz I,
Werten S,
Girbardt B,
Berndt L,
Delcea M,
Hinrichs W
Biochim Biophys Acta Proteins Proteom
:140404
(2020 Feb 27)
|
|
|
|
|
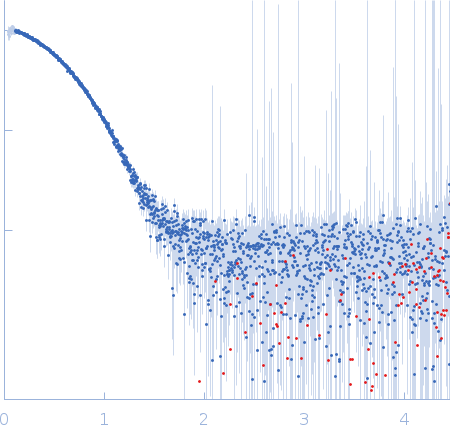
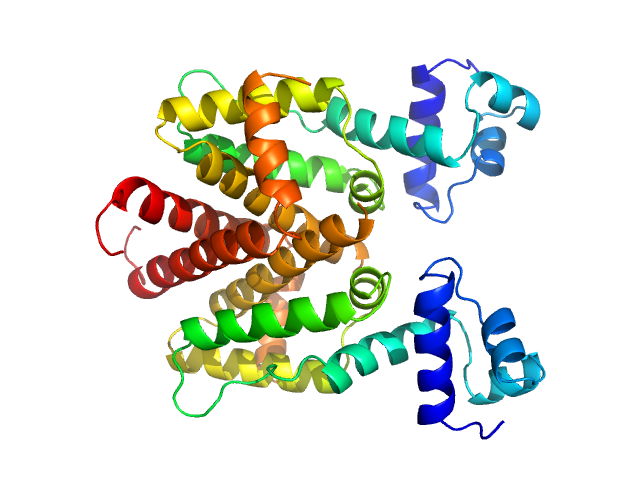
| Sample: |
Tetracycline repressor (class D) dimer, 47 kDa Escherichia coli protein
|
| Buffer: |
50 mM Tris/HCl 150 mM NaCl 10 mM MgCl2, pH: 8 |
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2013 Sep 23
|
|
| RgGuinier |
2.6 |
nm |
| Dmax |
7.7 |
nm |
| VolumePorod |
85 |
nm3 |
|
|
|
|
|
|
|
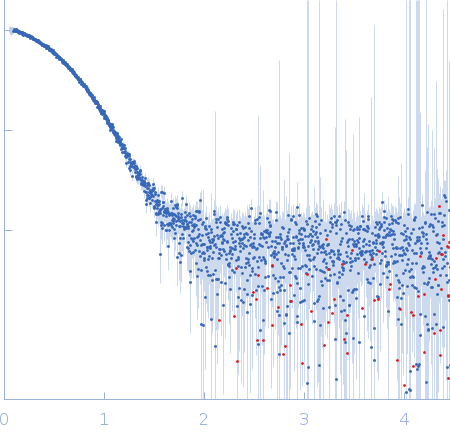
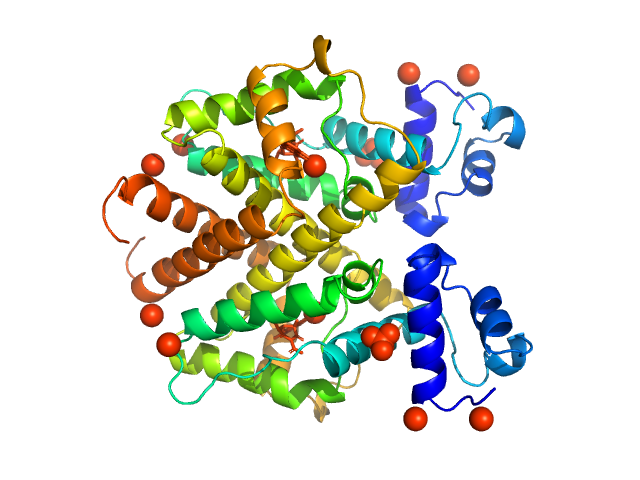
| Sample: |
Tetracycline repressor (class D) dimer, 47 kDa Escherichia coli protein
5a,6-anhydrotetracycline dimer, 1 kDa
|
| Buffer: |
50 mM Tris/HCl 150 mM NaCl 10 mM MgCl2, pH: 8 |
| Experiment: |
SAXS
data collected at EMBL P12, PETRA III on 2013 Sep 23
|
|
| RgGuinier |
2.6 |
nm |
| Dmax |
6.8 |
nm |
| VolumePorod |
77 |
nm3 |
|
|