Salt bridges at the subdomain interfaces of the adenylation domain and active-site residues of Mycobacterium tuberculosis
NAD +
-dependent DNA ligase A (MtbLigA) are important for the initial steps of nick-sealing activity
Afsar M,
Shukla A,
Kumar N,
Ramachandran R
Acta Crystallographica Section D Structural Biology
77(6)
(2021 May 14)
|
|
|
|

|
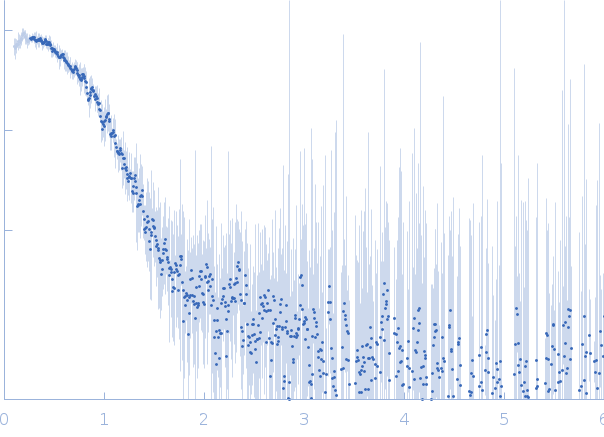
| Sample: |
DNA ligase A monomer, 38 kDa Mycobacterium tuberculosis protein
|
| Buffer: |
50 mM Tris-HCl, 200 mM NaCl, 2 mM β-mercaptoethanol, pH: 8 |
| Experiment: |
SAXS
data collected at Anton Paar SAXSpace, CSIR-Central Drug Research Institute on 2018 Sep 27
|
|
| RgGuinier |
2.4 |
nm |
| Dmax |
8.8 |
nm |
| VolumePorod |
69 |
nm3 |
|
|
|
|
|
|
|
| Sample: |
DNA ligase A monomer, 38 kDa Mycobacterium tuberculosis protein
|
| Buffer: |
50 mM Tris-HCl, 200 mM NaCl, 2 mM β-mercaptoethanol, pH: 8 |
| Experiment: |
SAXS
data collected at Anton Paar SAXSpace, CSIR-Central Drug Research Institute on 2018 Oct 6
|
|
| RgGuinier |
2.4 |
nm |
| Dmax |
6.2 |
nm |
| VolumePorod |
94 |
nm3 |
|
|
|
|
|
|

|
| Sample: |
DNA ligase A monomer, 37 kDa Mycobacterium tuberculosis protein
|
| Buffer: |
50 mM Tris-HCl 200 mM NaCl 2mM β-mercaptoethanol, pH: 8 |
| Experiment: |
SAXS
data collected at Anton Paar SAXSpace, CSIR-Central Drug Research Institute on 2019 May 21
|
|
| RgGuinier |
2.7 |
nm |
| Dmax |
8.9 |
nm |
| VolumePorod |
68 |
nm3 |
|
|