|
Synchrotron SAXS data from solutions of (POG)13 in 20 mM L-histidine, 138 mM NaCl, 2.7 mM KCL, pH 6 were collected on the B21 beam line at the Diamond Light Source (Didcot, UK) using a Pilatus 2M detector at a sample-detector distance of 4 m and at a wavelength of λ = 0.12 nm (I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). One solute concentration of 1.50 mg/ml was measured at 20°C. 60 successive 1 second frames were collected. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
The protein is N-terminal acetylated with a C-terminal amino group.
|
|
Ac-(POG)13-NH2
((POG)13)
|
| Mol. type |
|
Protein |
| Organism |
|
synthetic construct |
| Olig. state |
|
Trimer |
| Mon. MW |
|
5.1 kDa |
| Sequence |
|
FASTA |
| |
|
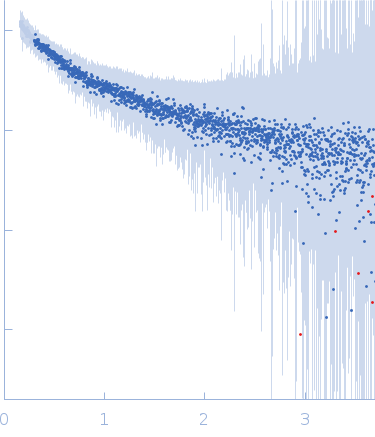 s, nm-1
s, nm-1