|
Synchrotron SAXS data from solutions of the di-domain acyl carrier protein of the seryltransferase from prodigiosin biosynthesis in 20 mM Tris, 5 mM DTT, pH 7, were collected on the BL1.3W beam line at the Synchrotron Light Research Institute (Nakhon Ratchasima, Thailand) using a CCD Rayonix SX165 detector at a sample-detector distance of 0.9 m and at a wavelength of λ = 0.131 nm (l(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). Solute concentrations ranging between 1.7 and 6.8 mg/ml were measured at 25°C. One 600 second frame was collected. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted. The low angle data collected at lower concentration were merged with the highest concentration high angle data to yield the final composite scattering curve.
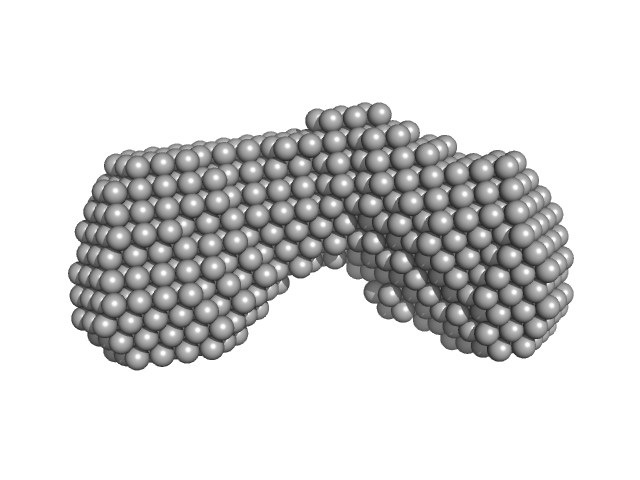
Two DAMMIF-derived dummy atom models are displayed. Top: The best-fit individual DAMMIF model and corresponding fit to the SAXS data. Bottom: The average spatial representation of the protein obtained after the alignment of multiple individual DAMMIF models combined with volume and bead-occupancy corrections (damfilt).
|
|
 s, nm-1
s, nm-1