|
Synchrotron SAXS data from solutions of LRSAM1 (amino acids 561-632) in 50 mM Tris-HCl pH 8.0, 150 mM NaCl, 1 mM TCEP, pH 8 were collected on the 4C beam line at the Pohang Accelerator Laboratory (Pohang, South Korea) using a Rayonix SX165 detector at a sample-detector distance of 3 m and at a wavelength of λ = 0.0734 nm (I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). One solute concentration of 16.20 mg/ml was measured at 20°C. Six successive 10 second frames were collected. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
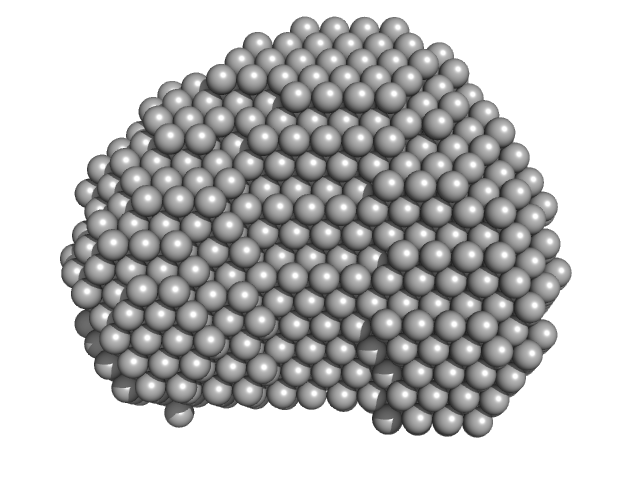
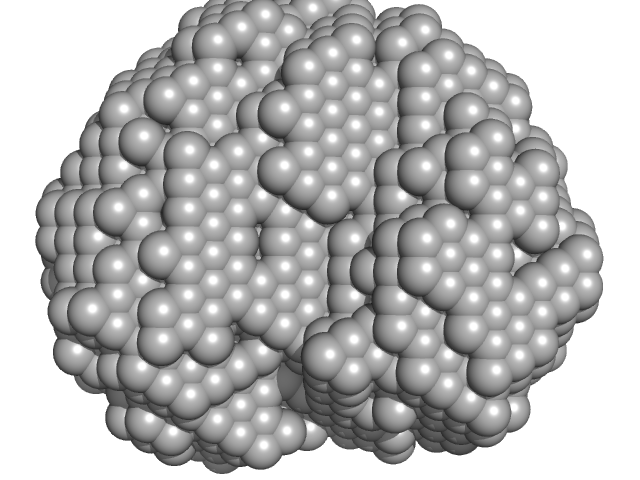
The models displayed show both the average-weighted bead-occupancy and volume-corrected representation of the protein calculated from the spatial alignment of a cohort of individual models (damfilt; top) and an individual model reconstruction (bottom) with the associated fit to the data.
|
|
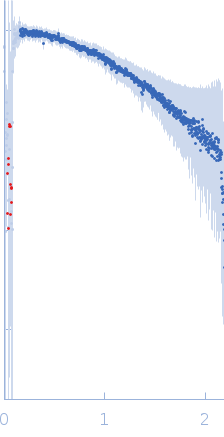 s, nm-1
s, nm-1