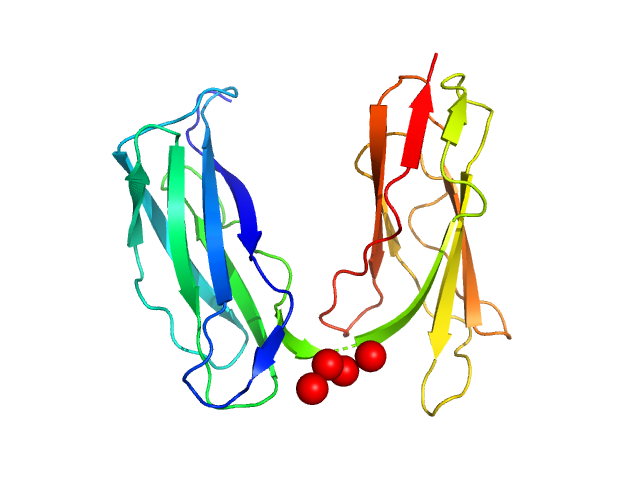
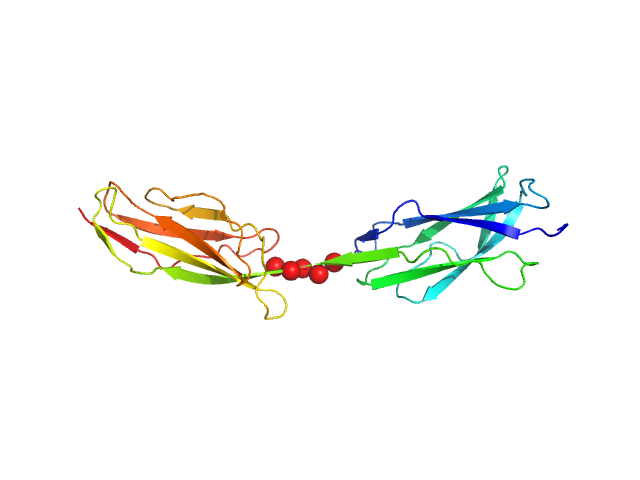
|
Synchrotron SAXS data from solutions of the intimin D0-D1 domain in 10 mM HEPES, pH 7.5 were collected on the EMBL P12 beam line at PETRA III (DESY, Hamburg, Germany) using a Pilatus 6M detector at a sample-detector distance of 4 m and at a wavelength of λ = 0.123981 nm (I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). Solute concentrations ranging between 1.1 and 12.4 mg/ml were measured . 20 successive 0.195 second frames were collected. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted. The low angle data collected at lower concentration were merged with the highest concentration high angle data to yield the final composite scattering curve.
The buffer before and the buffer after the sample did not match. Therefore, averaged data for buffer and for sample were scaled at high-s (starting from point nr 2000), resulting in the high noise at the high-s. All data analysis was focused on the low-s region (up to q=0.2 nm^-1). The models represent the ensemble state of the protein in solution.
|
|
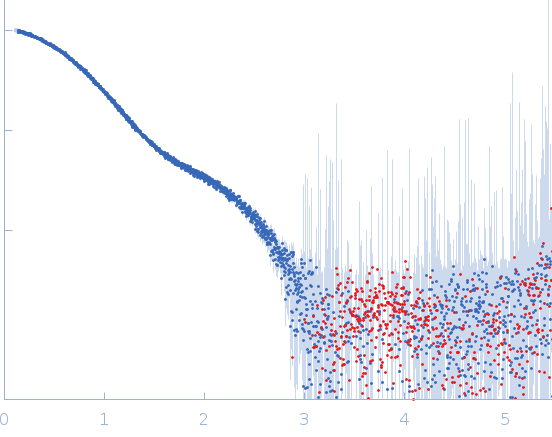 s, nm-1
s, nm-1
 Rg, nm
Rg, nm