|
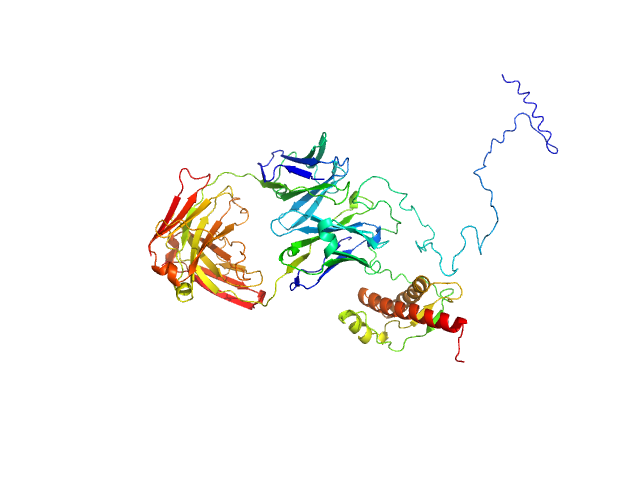
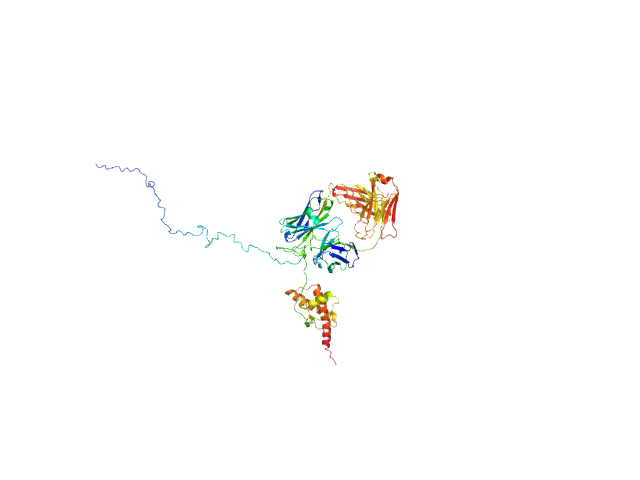
Synchrotron SAXS data from solutions of recombinant prion protein (89–230) in complex with Fab-P in 20 mM sodium acetate, 150 mM NaCl, pH 5.1 were collected on the BL4-2 beam line at the Stanford Synchrotron Radiation Lightsource (SSRL, Stanford, CA, USA) using a Rayonix MX225-HE detector at a sample-detector distance of 1.7 m and at a wavelength of λ = 0.113 nm (I(s) vs s, where s = 4πsin θ/λ, and 2θ is the scattering angle). Solute concentrations ranging between 1 and 3.7 mg/ml were measured at 9.8°C. 10 successive 1 second frames were collected. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted and the difference curves were scaled for protein concentration. The low angle data collected at lower concentration were merged with the highest concentration high angle data to yield the final composite scattering curve.
|
|
 s, nm-1
s, nm-1