|
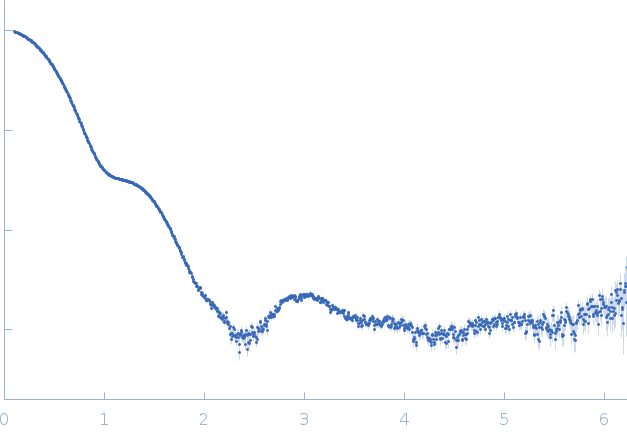
Synchrotron SAXS data from solutions of Protease 1 from Pyrococcus horikoshii (PhP1) in 20 mM Tris pH 7.5, 150 mM NaCl, were collected on the SWING beam line at SOLEIL (Saint-Aubin, France) using a AVIEX PCCD170170 detector at a sample-detector distance of 1.8 m and at a wavelength of λ = 0.1 nm (I(s) vs s, where s = 4πsinθ/λ, and 2θ is the scattering angle). One solute concentration of 6.00 mg/ml was measured at 15°C. 20 successive 1 second frames were collected. The data were normalized to the intensity of the transmitted beam and radially averaged; the scattering of the solvent-blank was subtracted.
The data displayed in this entry is that of hexameric Protease 1 without the addition of iohexol that acts as X-ray contrast variation agent. Refer to the full entry zip archive that contains additional SAXS data and model fits in the presence of 92-618 mM iohexol (https://pubchem.ncbi.nlm.nih.gov/compound/Iohexol).
|
|
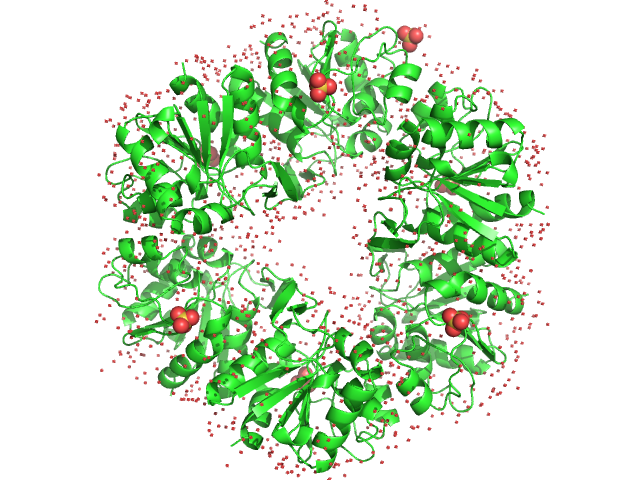
Deglycase PH1704
(PhP1)
|
| Mol. type |
|
Protein |
| Organism |
|
Pyrococcus horikoshii (strain ATCC 700860 / DSM 12428 / JCM 9974 / NBRC 100139 / OT-3) |
| Olig. state |
|
Hexamer |
| Mon. MW |
|
18.6 kDa |
| |
| UniProt |
|
O59413
(1-166)
|
| Sequence |
|
FASTA |
| |
|
 s, nm-1
s, nm-1