|
|
|
|

|
| Sample: |
NP-H5 pentamer, 200 kDa Escherichia coli protein
|
| Buffer: |
20 mM Pipes buffer 150 mM NaCl, pH: 7.5 |
| Experiment: |
SAXS
data collected at EMBL X33, DORIS III, DESY on 2007 Dec 3
|
A mechanism for histone chaperoning activity of nucleoplasmin: thermodynamic and structural models.
J Mol Biol 393(2):448-63 (2009)
Taneva SG, Bañuelos S, Falces J, Arregi I, Muga A, Konarev PV, Svergun DI, Velázquez-Campoy A, Urbaneja MA
|
| RgGuinier |
5.2 |
nm |
| Dmax |
16.7 |
nm |
| VolumePorod |
410 |
nm3 |
|
|
|
|
|
|

|
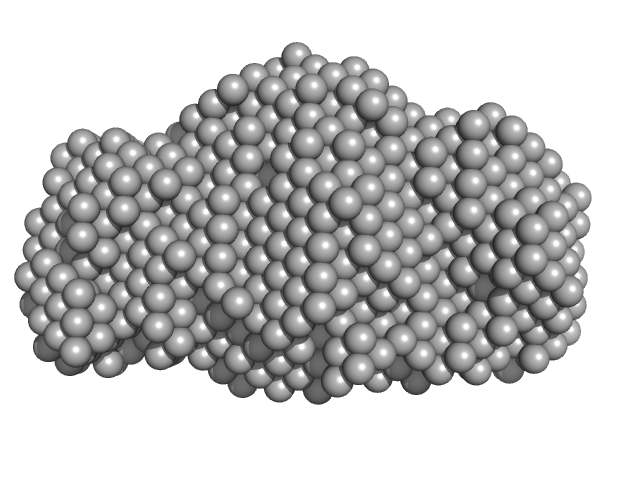
| Sample: |
NP-H2AH2B pentamer, 250 kDa Escherichia coli protein
|
| Buffer: |
20 mM Pipes buffer 150 mM NaCl, pH: 7.5 |
| Experiment: |
SAXS
data collected at EMBL X33, DORIS III, DESY on 2014 Dec 3
|
A mechanism for histone chaperoning activity of nucleoplasmin: thermodynamic and structural models.
J Mol Biol 393(2):448-63 (2009)
Taneva SG, Bañuelos S, Falces J, Arregi I, Muga A, Konarev PV, Svergun DI, Velázquez-Campoy A, Urbaneja MA
|
| RgGuinier |
4.7 |
nm |
| Dmax |
14.5 |
nm |
| VolumePorod |
470 |
nm3 |
|
|
|
|
|
|
|
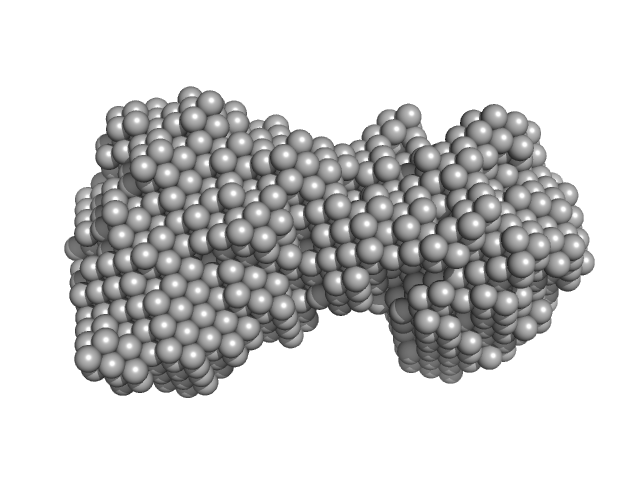
| Sample: |
Immunoglobulin- like filamin two-domain fragment 16-17 monomer, 19 kDa Escherichia coli protein
|
| Buffer: |
100 mM NaCl 10 mM dithiothreitol 20 mM Tris, pH: 8 |
| Experiment: |
SAXS
data collected at EMBL X33, DORIS III, DESY on 2007 Jun 18
|
Atomic structures of two novel immunoglobulin-like domain pairs in the actin cross-linking protein filamin.
J Biol Chem 284(37):25450-8 (2009)
Heikkinen OK, Ruskamo S, Konarev PV, Svergun DI, Iivanainen T, Heikkinen SM, Permi P, Koskela H, Kilpeläinen I, Ylänne J
|
| RgGuinier |
1.9 |
nm |
| Dmax |
6.0 |
nm |
| VolumePorod |
33 |
nm3 |
|
|
|
|
|
|
|
| Sample: |
Immunoglobulin- like filamin two-domain fragment 18-19 monomer, 20 kDa Escherichia coli protein
|
| Buffer: |
100 mM NaCl 10 mM dithiothreitol 20 mM Tris, pH: 8 |
| Experiment: |
SAXS
data collected at EMBL X33, DORIS III, DESY on 2007 Jun 18
|
Atomic structures of two novel immunoglobulin-like domain pairs in the actin cross-linking protein filamin.
J Biol Chem 284(37):25450-8 (2009)
Heikkinen OK, Ruskamo S, Konarev PV, Svergun DI, Iivanainen T, Heikkinen SM, Permi P, Koskela H, Kilpeläinen I, Ylänne J
|
| RgGuinier |
2.1 |
nm |
| Dmax |
6.5 |
nm |
| VolumePorod |
34 |
nm3 |
|
|
|
|
|
|

|
| Sample: |
Immunoglobulin- like filamin two-domain fragment 22-23 monomer, 19 kDa Escherichia coli protein
|
| Buffer: |
100 mM NaCl 10 mM dithiothreitol 20 mM Tris, pH: 8 |
| Experiment: |
SAXS
data collected at EMBL X33, DORIS III, DESY on 2007 Jun 18
|
Atomic structures of two novel immunoglobulin-like domain pairs in the actin cross-linking protein filamin.
J Biol Chem 284(37):25450-8 (2009)
Heikkinen OK, Ruskamo S, Konarev PV, Svergun DI, Iivanainen T, Heikkinen SM, Permi P, Koskela H, Kilpeläinen I, Ylänne J
|
| RgGuinier |
2.8 |
nm |
| Dmax |
9.0 |
nm |
| VolumePorod |
32 |
nm3 |
|
|
|
|
|
|
|
| Sample: |
Anaerobic nitric oxide reductase flavorubredoxin tetramer, 217 kDa Escherichia coli (strain … protein
|
| Buffer: |
50 mM Tris-HCl, 18% glycerol, pH: 7.5 |
| Experiment: |
SAXS
data collected at EMBL X33, DORIS III, DESY on 2006 Nov 19
|
Quaternary Structure of Flavorubredoxin as Revealed by Synchrotron Radiation Small-Angle X-Ray Scattering
Structure 16(9):1428-1436 (2008)
Petoukhov M, Vicente J, Crowley P, Carrondo M, Teixeira M, Svergun D
|
| RgGuinier |
4.1 |
nm |
| Dmax |
14.2 |
nm |
| VolumePorod |
297 |
nm3 |
|
|
|
|
|
|

|
| Sample: |
Der p21, allegren dimer, 26 kDa Escherichia coli protein
|
| Buffer: |
10 mM Hepes , pH: 7 |
| Experiment: |
SAXS
data collected at EMBL X33, DORIS III, DESY on 2006 Jul 24
|
Characterization of Der p 21, a new important allergen derived from the gut of house dust mites.
Allergy 63(6):758-67 (2008)
Weghofer M, Dall'Antonia Y, Grote M, Stöcklinger A, Kneidinger M, Balic N, Krauth MT, Fernández-Caldas E, Thomas WR, van Hage M, Vieths S, Spitzauer S, Horak F, Svergun DI, Konarev PV, Valent P, Thalhamer J, Keller W, Valenta R, Vrtala S
|
| RgGuinier |
2.7 |
nm |
| Dmax |
8.5 |
nm |
| VolumePorod |
50 |
nm3 |
|
|
|
|
|
|

|
| Sample: |
Cytochrome C monomer, 11 kDa Escherichia coli protein
Adrenodoxin monomer, 11 kDa Escherichia coli protein
|
| Buffer: |
20 mM HEPES 2 mM DTT, pH: 7.4 |
| Experiment: |
SAXS
data collected at EMBL X33, DORIS III, DESY on 2006 Jun 15
|
Dynamics in a pure encounter complex of two proteins studied by solution scattering and paramagnetic NMR spectroscopy.
J Am Chem Soc 130(20):6395-403 (2008)
Xu X, Reinle W, Hannemann F, Konarev PV, Svergun DI, Bernhardt R, Ubbink M
|
| RgGuinier |
2.1 |
nm |
| Dmax |
8.5 |
nm |
| VolumePorod |
42 |
nm3 |
|
|
|
|
|
|

|
| Sample: |
Cytochrome C dimer dimer, 22 kDa Escherichia coli protein
Adrenodoxin dimer dimer, 22 kDa Escherichia coli protein
|
| Buffer: |
20 mM HEPES 2 mM DTT, pH: 7.4 |
| Experiment: |
SAXS
data collected at EMBL X33, DORIS III, DESY on 2006 Jun 15
|
Dynamics in a pure encounter complex of two proteins studied by solution scattering and paramagnetic NMR spectroscopy.
J Am Chem Soc 130(20):6395-403 (2008)
Xu X, Reinle W, Hannemann F, Konarev PV, Svergun DI, Bernhardt R, Ubbink M
|
| RgGuinier |
2.9 |
nm |
| Dmax |
9.5 |
nm |
| VolumePorod |
64 |
nm3 |
|
|
|
|
|
|

|
| Sample: |
Filamin C 23-24 dimer, 40 kDa Escherichia coli protein
|
| Buffer: |
20 mM Tris-HCl 50 mM NaCl, pH: 8 |
| Experiment: |
SAXS
data collected at EMBL X33, DORIS III, DESY on 2005 Jun 17
|
Crystal structure of human filamin C domain 23 and small angle scattering model for filamin C 23-24 dimer.
J Mol Biol 368(4):1011-23 (2007)
Sjekloća L, Pudas R, Sjöblom B, Konarev P, Carugo O, Rybin V, Kiema TR, Svergun D, Ylänne J, Djinović Carugo K
|
| RgGuinier |
3.5 |
nm |
| Dmax |
13.0 |
nm |
| VolumePorod |
75 |
nm3 |
|
|