|
|
|
|

|
| Sample: |
NP-H5 pentamer, 200 kDa Escherichia coli protein
|
| Buffer: |
20 mM Pipes buffer 150 mM NaCl, pH: 7.5
|
| Experiment: |
SAXS
data collected at EMBL X33, DORIS III, DESY on 2007 Dec 3
|
A mechanism for histone chaperoning activity of nucleoplasmin: thermodynamic and structural models.
J Mol Biol 393(2):448-63 (2009)
Taneva SG, Bañuelos S, Falces J, Arregi I, Muga A, Konarev PV, Svergun DI, Velázquez-Campoy A, Urbaneja MA
|
| RgGuinier |
5.2 |
nm |
| Dmax |
16.7 |
nm |
| VolumePorod |
410 |
nm3 |
|
|
|
|
|
|

|
| Sample: |
NP-H2AH2B pentamer, 250 kDa Escherichia coli protein
|
| Buffer: |
20 mM Pipes buffer 150 mM NaCl, pH: 7.5
|
| Experiment: |
SAXS
data collected at EMBL X33, DORIS III, DESY on 2014 Dec 3
|
A mechanism for histone chaperoning activity of nucleoplasmin: thermodynamic and structural models.
J Mol Biol 393(2):448-63 (2009)
Taneva SG, Bañuelos S, Falces J, Arregi I, Muga A, Konarev PV, Svergun DI, Velázquez-Campoy A, Urbaneja MA
|
| RgGuinier |
4.7 |
nm |
| Dmax |
14.5 |
nm |
| VolumePorod |
470 |
nm3 |
|
|
|
|
|
|

|
| Sample: |
DH-PH module of PDZRhoGEF monomer, 41 kDa Homo sapiens protein
|
| Buffer: |
50 mM Tris-HCL 150 mM NaCl 1.0 mM DTT, pH: 7.5
|
| Experiment: |
SAXS
data collected at EMBL X33, DORIS III, DESY on 2008 Oct 30
|
The solution structure and dynamics of the DH-PH module of PDZRhoGEF in isolation and in complex with nucleotide-free RhoA.
Protein Sci 18(10):2067-79 (2009)
Cierpicki T, Bielnicki J, Zheng M, Gruszczyk J, Kasterka M, Petoukhov M, Zhang A, Fernandez EJ, Svergun DI, Derewenda U, Bushweller JH, Derewenda ZS
|
| RgGuinier |
2.9 |
nm |
| Dmax |
9.0 |
nm |
| VolumePorod |
60 |
nm3 |
|
|
|
|
|
|

|
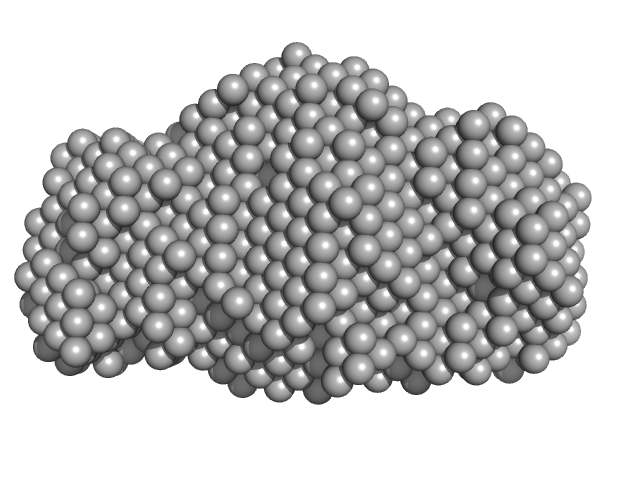
| Sample: |
Calmodulin monomer, 17 kDa Homo sapiens protein
Glutamate decarboxylase hexamer, 340 kDa Petunia x hybrida protein
|
| Buffer: |
50 mM HEPES 50 mM KCl, pH: 7.5
|
| Experiment: |
SAXS
data collected at EMBL X33, DORIS III, DESY on 2004 Nov 9
|
A common structural basis for pH- and calmodulin-mediated regulation in plant glutamate decarboxylase.
J Mol Biol 392(2):334-51 (2009)
Gut H, Dominici P, Pilati S, Astegno A, Petoukhov MV, Svergun DI, Grütter MG, Capitani G
|
| RgGuinier |
5.9 |
nm |
| Dmax |
23.0 |
nm |
| VolumePorod |
630 |
nm3 |
|
|
|
|
|
|
|
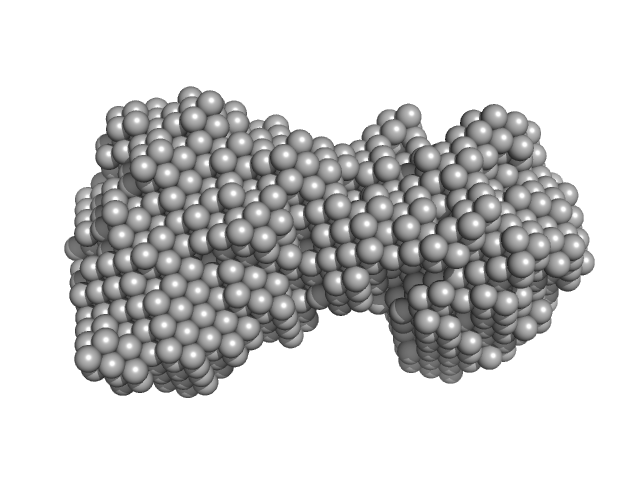
| Sample: |
immunoglobulin- like filamin two-domain fragment 16-17 monomer, 19 kDa Escherichia coli protein
|
| Buffer: |
100 mM NaCl 10 mM dithiothreitol 20 mM Tris, pH: 8
|
| Experiment: |
SAXS
data collected at EMBL X33, DORIS III, DESY on 2007 Jun 18
|
Atomic structures of two novel immunoglobulin-like domain pairs in the actin cross-linking protein filamin.
J Biol Chem 284(37):25450-8 (2009)
Heikkinen OK, Ruskamo S, Konarev PV, Svergun DI, Iivanainen T, Heikkinen SM, Permi P, Koskela H, Kilpeläinen I, Ylänne J
|
| RgGuinier |
1.9 |
nm |
| Dmax |
6.0 |
nm |
| VolumePorod |
33 |
nm3 |
|
|
|
|
|
|
|
| Sample: |
immunoglobulin- like filamin two-domain fragment 18-19 monomer, 20 kDa Escherichia coli protein
|
| Buffer: |
100 mM NaCl 10 mM dithiothreitol 20 mM Tris, pH: 8
|
| Experiment: |
SAXS
data collected at EMBL X33, DORIS III, DESY on 2007 Jun 18
|
Atomic structures of two novel immunoglobulin-like domain pairs in the actin cross-linking protein filamin.
J Biol Chem 284(37):25450-8 (2009)
Heikkinen OK, Ruskamo S, Konarev PV, Svergun DI, Iivanainen T, Heikkinen SM, Permi P, Koskela H, Kilpeläinen I, Ylänne J
|
| RgGuinier |
2.1 |
nm |
| Dmax |
6.5 |
nm |
| VolumePorod |
34 |
nm3 |
|
|
|
|
|
|

|
| Sample: |
immunoglobulin- like filamin two-domain fragment 22-23 monomer, 19 kDa Escherichia coli protein
|
| Buffer: |
100 mM NaCl 10 mM dithiothreitol 20 mM Tris, pH: 8
|
| Experiment: |
SAXS
data collected at EMBL X33, DORIS III, DESY on 2007 Jun 18
|
Atomic structures of two novel immunoglobulin-like domain pairs in the actin cross-linking protein filamin.
J Biol Chem 284(37):25450-8 (2009)
Heikkinen OK, Ruskamo S, Konarev PV, Svergun DI, Iivanainen T, Heikkinen SM, Permi P, Koskela H, Kilpeläinen I, Ylänne J
|
| RgGuinier |
2.8 |
nm |
| Dmax |
9.0 |
nm |
| VolumePorod |
32 |
nm3 |
|
|
|
|
|
|

|
| Sample: |
Peroxisomal targeting signal 1 receptor (C -terminal) monomer, 48 kDa Homo sapiens protein
Peroxisomal membrane protein PEX14 (N-terminal) monomer, 7 kDa Homo sapiens protein
PTS1-BP monomer, 14 kDa Homo sapiens protein
|
| Buffer: |
50 mM HEPES-KOH (pH 7.5), 100mM KCl, and 20mM TCEP, pH: 7.5
|
| Experiment: |
SAXS
data collected at EMBL X33, DORIS III, DESY on 2005 Oct 3
|
Solution structure of human Pex5.Pex14.PTS1 protein complexes obtained by small angle X-ray scattering.
J Biol Chem 284(37):25334-42 (2009)
Shiozawa K, Konarev PV, Neufeld C, Wilmanns M, Svergun DI
|
| RgGuinier |
2.9 |
nm |
| Dmax |
9.0 |
nm |
| VolumePorod |
110 |
nm3 |
|
|
|
|
|
|

|
| Sample: |
Peroxisomal targeting signal 1 receptor monomer, 71 kDa Homo sapiens protein
|
| Buffer: |
50 mM HEPES-KOH (pH 7.5), 100mM KCl, and 20mM TCEP, pH: 7.5
|
| Experiment: |
SAXS
data collected at EMBL X33, DORIS III, DESY on 2006 Feb 24
|
Solution structure of human Pex5.Pex14.PTS1 protein complexes obtained by small angle X-ray scattering.
J Biol Chem 284(37):25334-42 (2009)
Shiozawa K, Konarev PV, Neufeld C, Wilmanns M, Svergun DI
|
| RgGuinier |
5.0 |
nm |
| Dmax |
20.0 |
nm |
| VolumePorod |
181 |
nm3 |
|
|
|
|
|
|

|
| Sample: |
Peroxisomal targeting signal 1 receptor monomer, 71 kDa Homo sapiens protein
Peroxisomal membrane protein PEX14 monomer, 7 kDa Homo sapiens protein
PTS1-BP monomer, 13 kDa Homo sapiens protein
|
| Buffer: |
50 mM HEPES-KOH (pH 7.5), 100mM KCl, and 20mM TCEP, pH: 7.5
|
| Experiment: |
SAXS
data collected at EMBL X33, DORIS III, DESY on 2006 Mar 18
|
Solution structure of human Pex5.Pex14.PTS1 protein complexes obtained by small angle X-ray scattering.
J Biol Chem 284(37):25334-42 (2009)
Shiozawa K, Konarev PV, Neufeld C, Wilmanns M, Svergun DI
|
| RgGuinier |
6.0 |
nm |
| Dmax |
20.0 |
nm |
| VolumePorod |
267 |
nm3 |
|
|